


In the complex realm of clinical research, the role of a study start-up specialist is crucial, acting as the vital link between regulatory compliance, project management, and ethical considerations. This article explores the nine essential skills these specialists must master to effectively navigate the intricacies of clinical trials. Readers will discover how proficiency in areas such as regulatory knowledge, financial acumen, and teamwork not only boosts the efficiency of research processes but also protects participant rights and data integrity. As the landscape of clinical research continues to evolve, one question stands out: what strategies can study start-up specialists implement to tackle emerging challenges and ensure the success of their trials?
A study start up specialist is essential in clinical research, necessitating a thorough understanding of regulations established by the FDA, EMA, and local authorities. This expertise encompasses a thorough grasp of Good Clinical Practice (GCP) guidelines, which prioritize participant safety and data integrity. GCP mandates that all clinical study participants provide informed consent, ensuring they are fully aware of the study's purpose, procedures, risks, and benefits.
Staying informed about regulatory changes is essential, as nearly 80% of studies fail to meet enrollment timelines, often due to non-compliance with evolving standards. By adhering to GCP, specialists can enhance the credibility of research data, facilitating acceptance by regulatory authorities worldwide. This knowledge not only safeguards participant rights but also streamlines the path to market for new therapies, making it a vital skill for success in research involving trials.
At bioaccess, we offer extensive study oversight services, including:
This holistic approach ensures that our clients, particularly Medtech, Biopharma, and Radiopharma startups, can efficiently accelerate their clinical trials and achieve regulatory approval with the support of a study start up specialist. Collaboration is key in navigating the complexities of clinical research, and we are here to support you every step of the way.

A study start up specialist plays a crucial role in clinical research, as it requires strong project coordination skills to effectively oversee essential activities such as site selection, budget oversight, and timeline development. By leveraging advanced project coordination tools and methodologies, they can meticulously monitor progress, manage risks, and ensure that all team members are aligned with the study objectives. Establishing clear milestones and conducting regular project status reviews allows them to proactively address potential issues.
As Benjamin Franklin wisely noted, 'By failing to prepare, you are preparing to fail,' underscoring the significance of comprehensive planning in trial oversight. Entities that invest in organized project oversight experience projects that are 2.5 times more successful, highlighting the critical role of efficient coordination in achieving research objectives. In 2025, the incorporation of innovative project coordination tools will be essential, as 82% of organizations now utilize such resources to enhance efficiency and collaboration.
Ultimately, successful site selection and budget management hinge on a well-coordinated strategy, with a study start up specialist ensuring that research studies are conducted seamlessly and efficiently. Are you prepared to elevate your project oversight to meet these emerging challenges?
A study start up specialist plays a crucial role in clinical trials, as they require mastery of both verbal and written communication to effectively engage stakeholders. This involves:
Regular updates and feedback sessions are essential for addressing concerns early, ensuring stakeholders remain informed about study progress and any changes.
Employing tools like newsletters, project coordination software, and digital platforms significantly enhances communication processes. These tools simplify the monitoring of progress and foster collaboration among stakeholders. In fact, organizations that prioritize stakeholder involvement are five times more likely to achieve high performance in research. This statistic underscores the importance of effective communication in driving successful outcomes.
Furthermore, bioaccess offers extensive management services for studies, including the expertise of a study start up specialist in:
These services are vital for the successful execution of research, highlighting the need for collaboration and strategic planning in the Medtech landscape. As the industry evolves, embracing collaborative working environments will be key to overcoming challenges and achieving research excellence.

The role of a Study Start Up Specialist is crucial in the early identification of potential challenges in clinical research, including recruitment difficulties and regulatory hurdles. By implementing a systematic problem-solving approach-comprising problem definition, root cause analysis, and actionable solution development-risks can be significantly mitigated. For instance, studies indicate that locations with adequate infrastructure for trials are 41% more effective in patient enrollment, underscoring the necessity for strategic planning.
Moreover, bioaccess™ offers essential services like regulatory approval, research site activation, and patient recruitment, which are vital in overcoming these challenges. Fostering a culture of open communication within the team not only encourages members to voice concerns but also enhances collaborative efforts in finding solutions. This proactive stance is particularly important as the healthcare landscape shifts towards more personalized and technology-driven models, demanding adaptability and innovation in research practices.
To enhance success, it is essential for study start up specialists to consistently evaluate their strategies and remain adaptable to the ever-changing environment of research studies. By doing so, they can ensure that they are not only addressing current challenges but also positioning themselves for future opportunities.

A study start up specialist is essential in clinical research, especially in prioritizing strong data handling practices to ensure the accuracy and security of clinical trial data. This responsibility involves crafting a comprehensive data handling plan that outlines data collection methods, storage solutions, and validation processes. Regular audits and data quality checks are essential for early identification of discrepancies, thereby maintaining data reliability throughout the research process.
In 2025, the integration of electronic data capture (EDC) systems has proven to reduce entry mistakes by up to 50%, significantly enhancing data integrity. Moreover, research indicates that around 30% of medical studies fail due to inaccuracies, underscoring the critical need for careful information oversight. Achieving high data reliability is essential for fostering trust in medical findings and ensuring patient safety. For instance, over 61% of cardiovascular experiments have been found to overestimate expected event rates, which highlights the necessity for accurate estimations to avoid misleading conclusions and high failure rates in pharmaceutical development.
Furthermore, monitoring expenses could be lowered by between 43% and 63% in large studies utilizing risk-based monitoring (RBM), showcasing the advantages of technology in data oversight. By applying these strategies, along with thorough clinical trial oversight services such as:
a study start up specialist can effectively protect the accuracy and security of clinical research data. This proactive approach not only enhances the integrity of clinical trials but also contributes to the overall success of medical research.

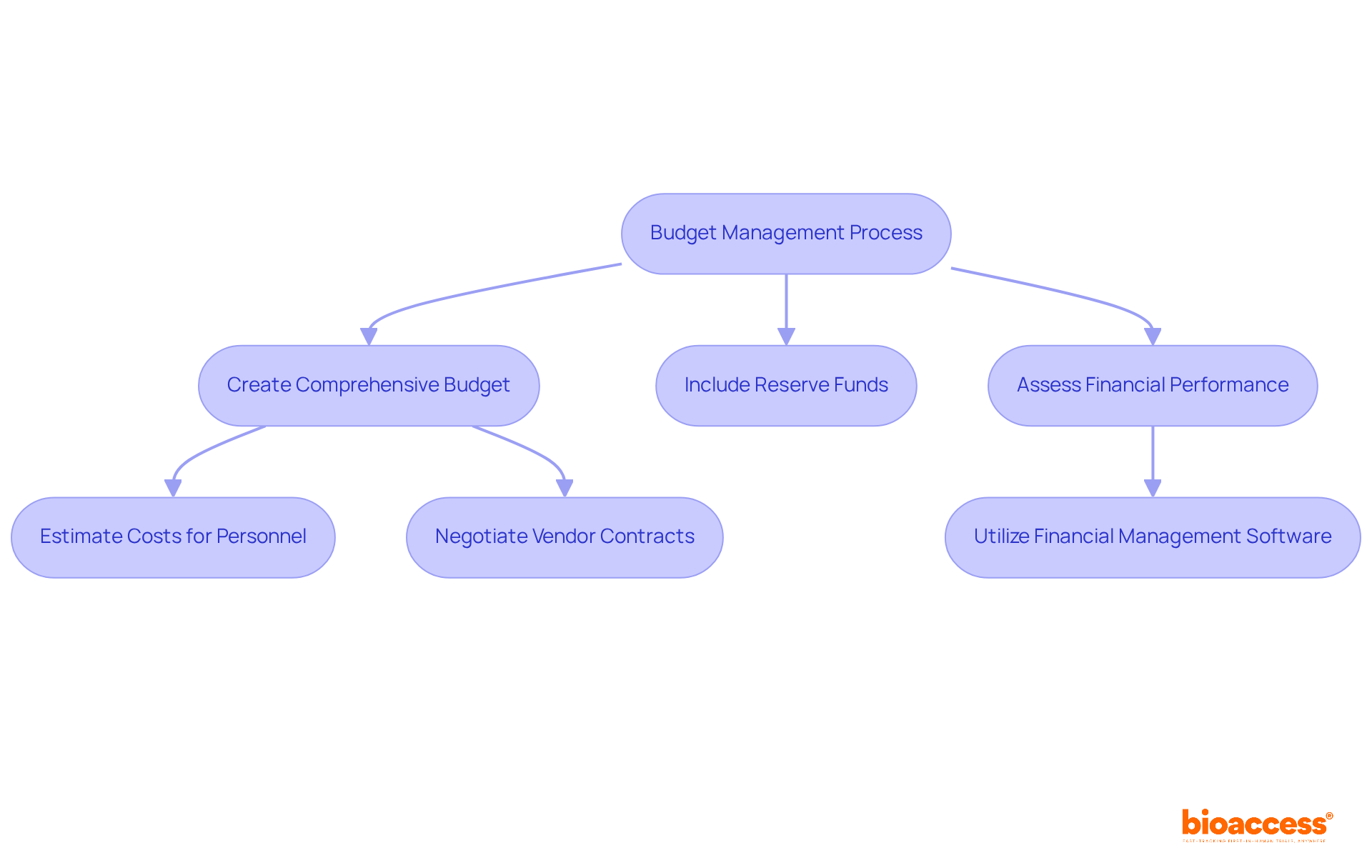
A study start up specialist is crucial in clinical research, necessitating strong financial acumen, especially in budget planning and financial management. Accurately estimating costs for various study components - such as personnel, site fees, and regulatory compliance - is essential, as these factors can significantly impact the overall budget. Did you know that 60% of research projects exceed their budgets by an average of 30%? This statistic underscores the importance of negotiating contracts with vendors to secure favorable terms that can help reduce expenses.
Tracking spending throughout the study is crucial. Utilizing advanced financial management software can streamline budget tracking and reporting, enabling timely adjustments to stay within budgetary limits. For a study start up specialist, optimal strategies for financial planning in research studies involve:
As we look ahead to 2025, with operational expenses on the rise, efficient financial planning will be more essential than ever to guarantee the sustainability and success of medical studies.
A study start up specialist must prioritize adaptability to effectively navigate the ever-evolving landscape of clinical research. With a staggering 76% of research trials now requiring at least one amendment, the ability to revise timelines, adjust recruitment strategies, and alter designs is not just beneficial - it's essential.
Proactive engagement with stakeholders, including patient advisory boards, can significantly mitigate unnecessary amendments, which often arise from inadequate initial planning. By fostering a culture of open feedback and continuous improvement, a study start up specialist can facilitate smoother transitions and enhance overall study success.
As John C. Maxwell aptly observes, 'The leader adjusts the sails,' underscoring the critical role of adaptability in achieving desired outcomes in a constantly shifting healthcare environment.

A study start up specialist plays a crucial role in prioritizing ethical considerations throughout the clinical research process, especially in terms of informed consent and participant confidentiality. Ethical practices are not merely formalities; they are essential components that require clear communication and a thorough understanding of the study's nature, risks, and benefits.
For instance, recent studies reveal that simplifying consent documents to an eighth-grade reading level significantly boosts comprehension, ensuring participants are fully informed before agreeing to participate. Yet, challenges remain, especially in developing countries, where illiteracy and language diversity complicate the informed consent process.
Equally important is the maintenance of participant confidentiality. Researchers must adopt robust data protection measures, such as anonymizing participant information and securing consent for data usage. The integration of multimedia tools in the informed consent process has proven effective in enhancing understanding and retention of information, particularly among participants with varying literacy levels. For example, audio-visual aids can clarify complex concepts and foster greater participant engagement.
As we look ahead to 2025, informed consent practices are evolving, with a strong emphasis on transparency and participant autonomy. Consistent training on ethical practices and fostering a culture of integrity within the research team are vital for reinforcing the significance of ethical behavior in trials. This focus on ethics is particularly relevant given the historical mistreatments in research that have shaped current standards. By prioritizing these elements, the study start up specialist can ensure that research is conducted ethically, ultimately enhancing participant trust and the overall integrity of the research process.

A study start up specialist must prioritize strong teamwork skills to effectively collaborate with investigators, site staff, and other stakeholders. Establishing clear roles and responsibilities is essential, as it fosters accountability and clarity within the team. Encouraging transparent dialogue is equally significant; it promotes the exchange of ideas and concerns, leading to creative solutions and improved outcomes. Frequent team gatherings and team-building exercises not only enhance relationships but also cultivate a cooperative culture that is vital for success in research studies.
In 2025, collaboration among various research groups is more crucial than ever. Evidence shows that inclusive teams make superior business decisions 87% of the time. This diversity enriches the research process and ensures that the needs of a broader patient population are considered. As Sundar Pichai noted, a diverse mix of voices leads to better discussions, decisions, and outcomes.
Best practices for collaborating with diverse research teams include:
By leveraging the strengths of varied team members, a study start up specialist can foster innovation and enhance the effectiveness of research studies. Bioaccess enhances this collaborative effort by offering comprehensive study management services, including:
These services streamline the research process and contribute to driving global health improvement through international collaboration and innovation in Medtech.

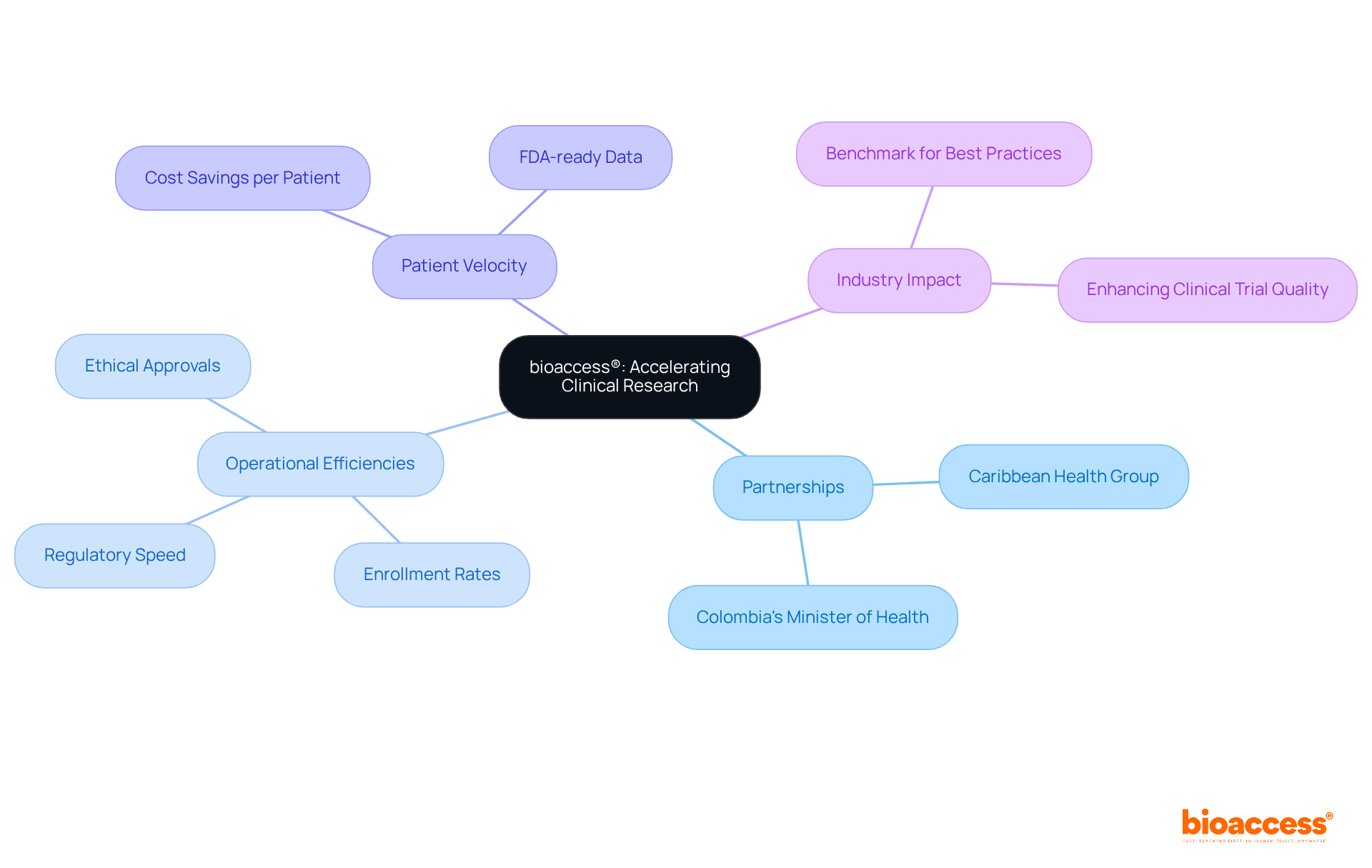
bioaccess® exemplifies a model for accelerating medical research through innovative strategies and streamlined processes. By partnering with Caribbean Health Group and receiving assistance from Colombia's Minister of Health during a meeting on March 29, 2019, in Miami, FL, bioaccess® is establishing Barranquilla as a prime location for medical studies in Latin America. Leveraging regional advantages such as regulatory speed and diverse patient pools, bioaccess® achieves ethical approvals within 4-6 weeks and boasts enrollment rates that are 50% faster than traditional markets. This operational agility not only shortens timelines but also enhances the overall quality of clinical trials, setting a benchmark for best practices in the industry.
Additionally, with the 'Patient Velocity' feature, bioaccess® allows study start-up specialists to achieve $25K savings per patient through FDA-ready data. This provides valuable lessons for those who are study start-up specialists aiming to enhance their own processes. As the Medtech landscape evolves, collaboration and innovative approaches like those of bioaccess® are crucial in addressing key challenges and driving progress in clinical research.
In the realm of clinical research, a study start-up specialist plays a pivotal role, embodying a multifaceted skill set that is essential for the success of clinical trials. Their mastery in regulatory knowledge, project management, communication, problem-solving, data management, financial acumen, adaptability, ethical considerations, and teamwork empowers them to navigate the complexities of study initiation. This comprehensive expertise not only safeguards participant rights but also enhances the integrity and efficiency of clinical research processes.
Key insights throughout this article have underscored the importance of each skill:
As the landscape of clinical research evolves, the significance of these skills becomes increasingly apparent. Embracing a culture of collaboration and innovation is essential for study start-up specialists to thrive. By investing in these vital skills, professionals can enhance their own capabilities and contribute to the advancement of medical research and the development of groundbreaking therapies. The future of clinical trials hinges on the dedication and expertise of those committed to excellence in study start-up.
What is the role of a study start up specialist in clinical research?
A study start up specialist is essential in clinical research, requiring a thorough understanding of regulations from the FDA, EMA, and local authorities. They oversee activities such as site selection, budget oversight, and timeline development to ensure studies are conducted efficiently and in compliance with regulations.
What are Good Clinical Practice (GCP) guidelines?
Good Clinical Practice (GCP) guidelines prioritize participant safety and data integrity in clinical trials. They mandate that all participants provide informed consent, ensuring they understand the study's purpose, procedures, risks, and benefits.
Why is staying informed about regulatory changes important in clinical research?
Staying informed about regulatory changes is crucial because nearly 80% of studies fail to meet enrollment timelines due to non-compliance with evolving standards. Adhering to GCP enhances the credibility of research data and facilitates acceptance by regulatory authorities.
What services does bioaccess offer to support clinical trials?
Bioaccess offers extensive study oversight services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project coordination, and reporting to help clients accelerate their clinical trials and achieve regulatory approval.
How does project management contribute to the success of clinical trials?
Effective project management, including strong coordination skills and the use of advanced project tools, helps monitor progress, manage risks, and ensure alignment with study objectives. Organized project oversight leads to higher success rates in research projects.
What tools can enhance communication among stakeholders in clinical trials?
Tools such as newsletters, project coordination software, and digital platforms can significantly enhance communication processes, allowing for regular updates and feedback sessions to keep stakeholders informed and engaged.
What is the impact of stakeholder involvement on research performance?
Organizations that prioritize stakeholder involvement are five times more likely to achieve high performance in research, highlighting the importance of effective communication in driving successful outcomes.
What is the significance of collaboration in clinical research?
Collaboration is key in navigating the complexities of clinical research. Embracing collaborative working environments is essential for overcoming challenges and achieving research excellence in the evolving Medtech landscape.