


The key elements of an effective clinical trial protocol encompass:
These elements are crucial in ensuring the integrity and success of the study. A well-structured protocol not only facilitates compliance with regulatory standards but also enhances participant safety. Ultimately, this leads to more efficient and credible clinical research outcomes, underscoring the importance of meticulous planning in clinical trials.
A well-structured clinical trial protocol serves as the backbone of successful medical research, providing a roadmap that guides researchers through complex studies while ensuring compliance and participant safety. By examining the nine key elements that contribute to an effective protocol, stakeholders can unlock the potential for innovative discoveries and enhanced patient outcomes. Yet, amid the intricacies of regulatory requirements and ethical considerations, how can researchers design protocols that not only meet established standards but also adapt to the evolving landscape of clinical trials?
A clinical research plan serves as a comprehensive document that delineates the objectives, design, methodology, statistical considerations, and operational aspects of a clinical study. This document acts as a guiding framework for the research, ensuring that all participants align with the trial's purpose and procedures. A clearly established framework is vital for maintaining research integrity and ensuring compliance with regulatory standards; non-adherence can lead to project delays, data invalidation, or regulatory penalties.
Typically, a comprehensive plan includes sections on:
For example, in a recent oncology study, the guidelines were meticulously crafted to evaluate overall survival in patients with advanced non-small cell lung cancer. This case underscores the significance of precise eligibility criteria and detailed procedures for data collection and safety assessments.
Moreover, professional insights reveal the critical need to include older adults in medical studies, as they represent a substantial demographic in cancer diagnoses. Despite this, only 25 percent of participants in cancer-focused studies are aged 65 and older, highlighting a gap that well-organized guidelines can effectively address.
Incorporating elements such as informed consent procedures, safety oversight, and risk management strategies, research guidelines not only protect participants but also enhance the credibility of the study. For instance, the implementation of an electronic data capture (EDC) system with automated validation checks has proven effective in addressing data entry inconsistencies, thereby safeguarding study integrity.
Ultimately, the significance of a comprehensive clinical trial protocol cannot be overstated, as it is foundational to the success of clinical research, ensuring that innovative discoveries can effectively reach patients.
A well-crafted research proposal is essential for articulating the project's objectives, which must adhere to the SMART criteria:
By outlining objectives in this manner, researchers maintain focus throughout the research process, ensuring that all stakeholders—including regulatory bodies and potential participants—comprehend the intended outcomes. This clarity simplifies the research design and enhances communication, fostering a collective understanding of the research objectives.
For example, a research objective may seek to assess the impact of dietary factors, particularly protein consumption, on the muscular health of participants or evaluate the relationship between sedentary habits and muscle atrophy within a specific demographic. Current trends underscore the significance of clear goals, as they facilitate the efficient evaluation of advancements and results, ultimately contributing to the success of medical studies.
Furthermore, distinguishing between research aims, which provide a broad statement of purpose, and specific objectives is crucial for clarity in research proposals.
The review process of the clinical trial protocol is critical in clinical research, encompassing a thorough assessment of research design, methodology, and ethical considerations. This process typically involves internal reviews by the research team, complemented by external evaluations from ethics committees and regulatory bodies. Given the convoluted regulatory requirements that early-phase clinical studies encounter, ensuring compliance with the clinical trial protocol, Good Clinical Practice (GCP) guidelines, and local regulations is essential for maintaining the quality and integrity of the study.
bioaccess® plays a pivotal role in managing these complexities, offering comprehensive assistance that includes:
A well-organized clinical trial protocol not only aids in identifying and resolving potential issues before testing begins but also safeguards participant welfare and data integrity. Furthermore, it accelerates approval processes for startups, underscoring the importance of collaboration in navigating the Medtech landscape.
In summary, the collaboration between research teams and bioaccess® is vital in addressing key challenges in clinical research. By leveraging expertise and resources, stakeholders can enhance the efficiency and effectiveness of their studies, ultimately driving innovation in the field.
When developing a research study plan, several key factors must be prioritized to ensure its effectiveness. A clinical trial protocol is a document detailing how a clinical trial will be conducted, including objectives, design, methodology, and safety measures. Clarity, conciseness, and comprehensiveness are paramount. The procedure should be logically organized, featuring clear headings and subheadings that facilitate easy navigation. Key components to include are:
Involving interdisciplinary teams throughout the writing process is essential, as their varied viewpoints and knowledge can greatly improve the project's strength. For instance, a multi-disciplinary team method has been demonstrated to yield more comprehensive and efficient procedures, ensuring that all elements of the study are carefully addressed. Moreover, integrating perspectives from healthcare professionals, data analysts, and project leaders can enhance the quality of information and reduce the necessity for revisions, ultimately enabling more seamless execution of the study.
Furthermore, bioaccess provides extensive management services for research projects, encompassing:
These services are essential for ensuring that guidelines meet regulatory standards. Sample size determinations should be grounded in the main aim of the study, as this is crucial for ensuring the research can identify significant differences. By highlighting these best practices, researchers can develop guidelines that not only satisfy regulatory standards but also prioritize participant safety and data integrity.
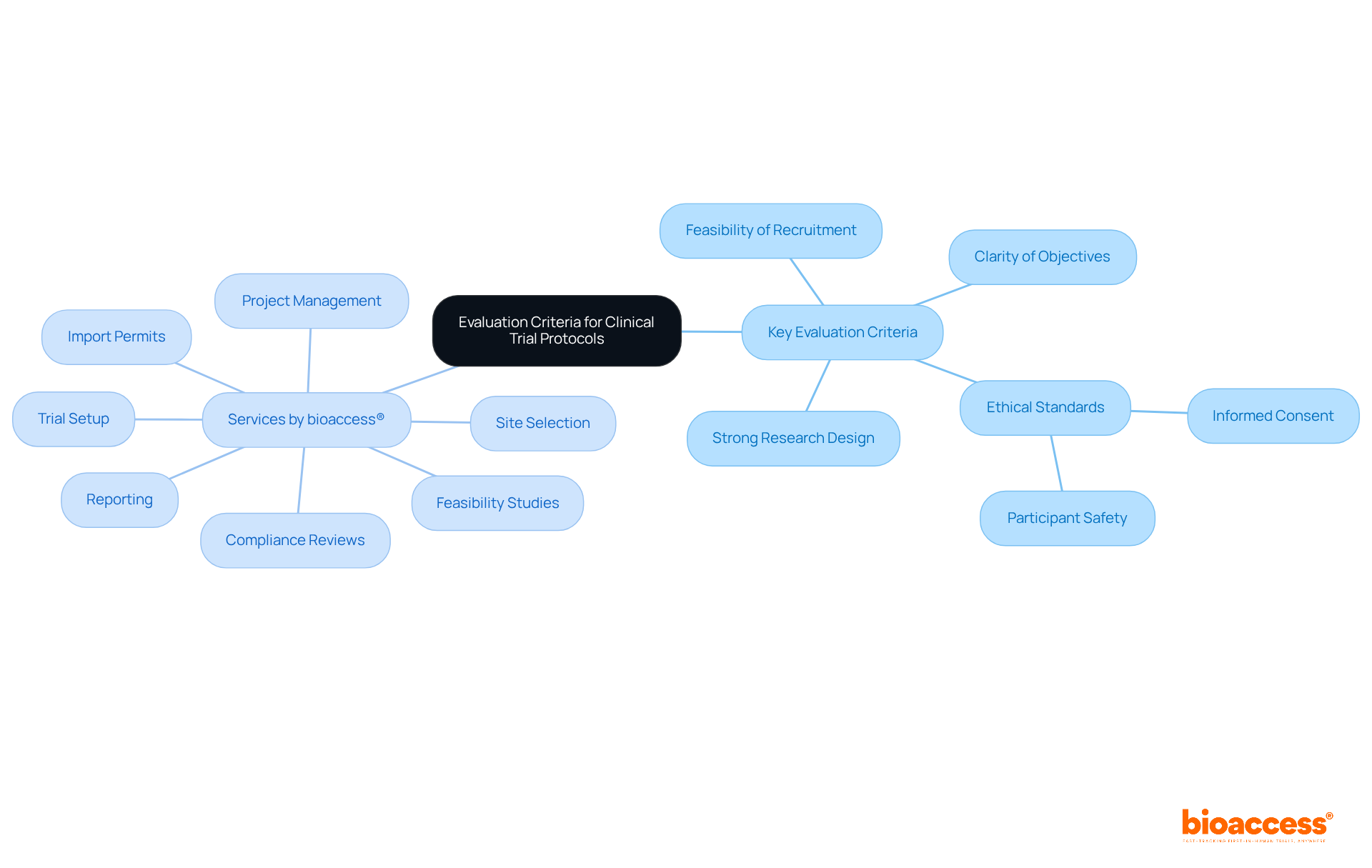
An effective clinical trial protocol must satisfy several key evaluation criteria to ensure its success. These encompass:
Given the challenges faced by Medtech and Biopharma startups in recruiting patients—often due to reluctance, eligibility issues, or overwhelming options—the clinical trial protocol should include strategies to enhance recruitment feasibility. It must clearly articulate the research question and outline a comprehensive plan for data collection and analysis, in accordance with ICH E3, E6(R2), E8(R2), E9(R1), and E17 guidelines.
Furthermore, it must reflect a thorough understanding of the regulatory landscape, incorporating measures for participant safety and data integrity. Regular assessments against these criteria are crucial to maintain alignment with the project's goals and improve the chances of favorable results.
Additionally, bioaccess® offers comprehensive services for managing the clinical trial protocol, including:
These services are crucial for navigating the complexities of clinical trials. Training for investigators on recognizing significant procedural deviations is also vital for ensuring adherence and protecting participant rights.
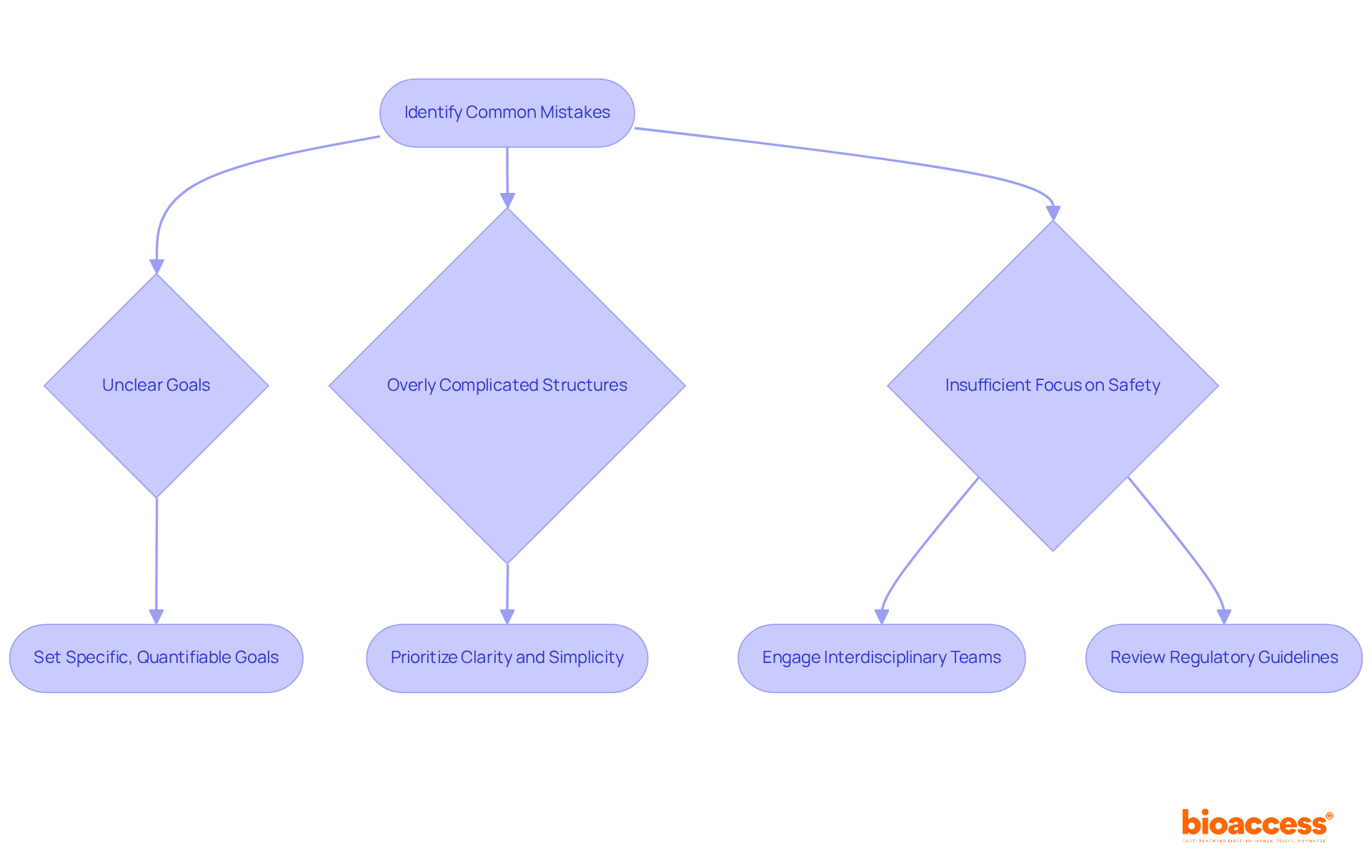
Frequent errors in document creation often stem from unclear goals, overly complicated structures, and insufficient focus on participant safety. Researchers frequently overlook the critical importance of engaging interdisciplinary teams, neglect regulatory requirements, and fail to provide adequate detail in their methodology.
To mitigate these issues, prioritizing clarity and simplicity in protocol development is essential. Engaging with colleagues for feedback can illuminate potential weaknesses, while a thorough review of regulatory guidelines ensures compliance. Furthermore, concentrating on specific, quantifiable goals can avert the drawbacks associated with vagueness, which can lead to misunderstandings and inefficiencies in implementation.
Research has demonstrated that unclear goals can significantly obstruct the efficiency of medical experiments, underscoring the necessity for precise formulation. By adopting a streamlined approach and actively involving diverse expertise—including comprehensive management services such as feasibility assessments, setup, and ethics committee approval—researchers can enhance the quality and efficacy of their research procedures.
It is crucial to navigate the intricacies of compliance evaluations and setup processes, especially in areas regulated by authorities such as INVIMA in Colombia, which oversees medical device regulations and classifications.
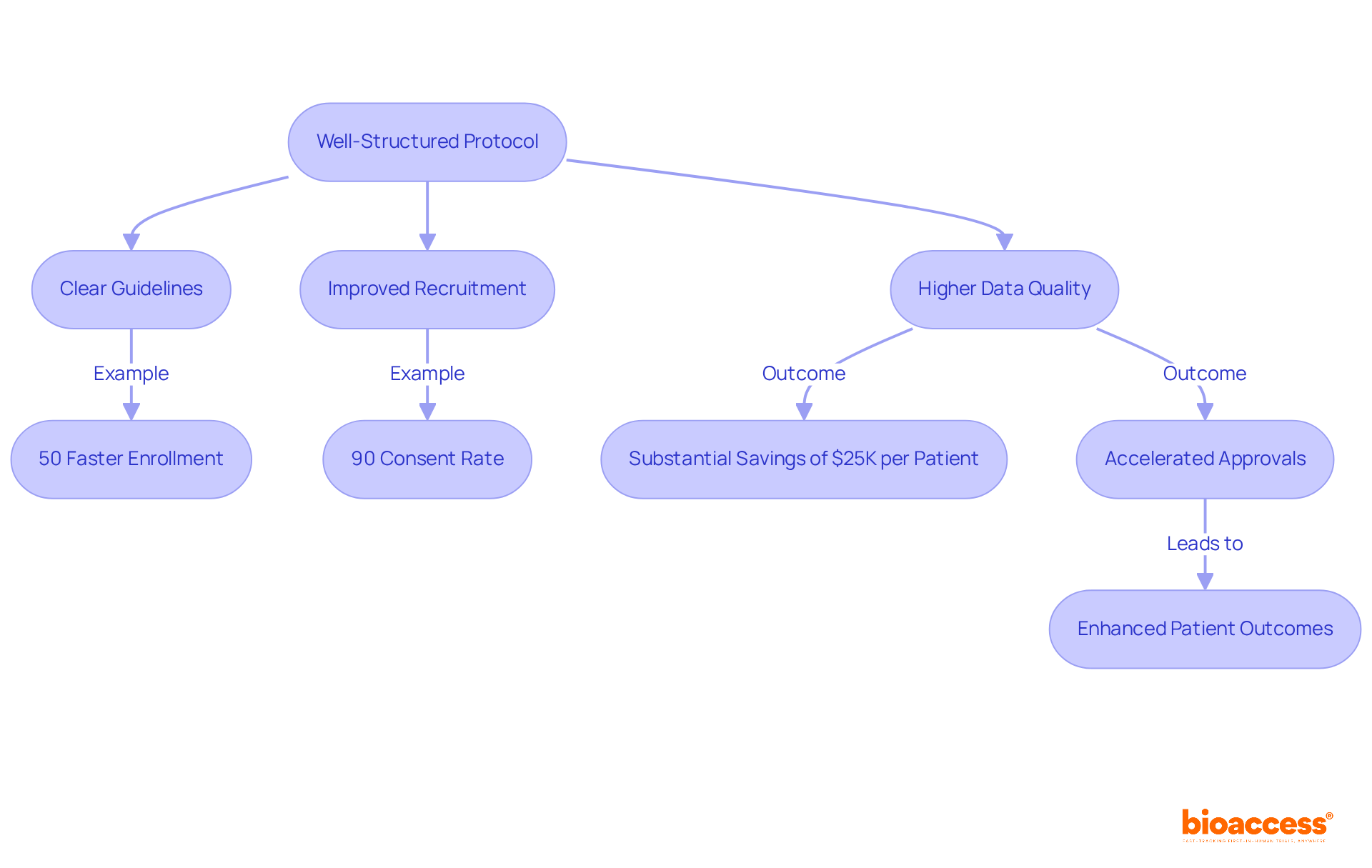
A well-organized clinical trial protocol significantly enhances research efficiency by providing clear guidelines that align all team members with the study's objectives and procedures. This clarity minimizes confusion, leading to improved participant recruitment and higher data quality. For instance, bioaccess® enables treatment-naive cardiology or neurology cohorts to enroll 50% faster than traditional Western sites, achieving substantial savings of $25K per patient with FDA-ready data—ensuring no rework and no delays.
The RECOVERY study exemplified this, demonstrating that a straightforward procedure facilitated rapid patient enrollment, reaching over 70 participants within two weeks and an impressive 90% consent rate from patients. Furthermore, thorough guidelines within the clinical trial protocol foster more seamless engagements with regulatory authorities and ethics boards, thereby accelerating approvals and optimizing the process.
Master protocols, which permit adaptive study designs, have been shown to shorten timelines by optimizing patient enrollment and allowing concurrent assessment of multiple treatments as part of a clinical trial protocol. This innovative approach not only accelerates drug development but also enhances the likelihood of identifying effective therapies, ultimately benefiting patient outcomes.
As Dr. Raha West observed, 'The simplicity of the RECOVERY study and its cooperative framework demonstrated that integrating research into regular medical practice can swiftly address vital inquiries.' Additionally, the partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a premier location for medical investigations in Latin America, supported by Colombia's Minister of Health, further improving the environment for research.
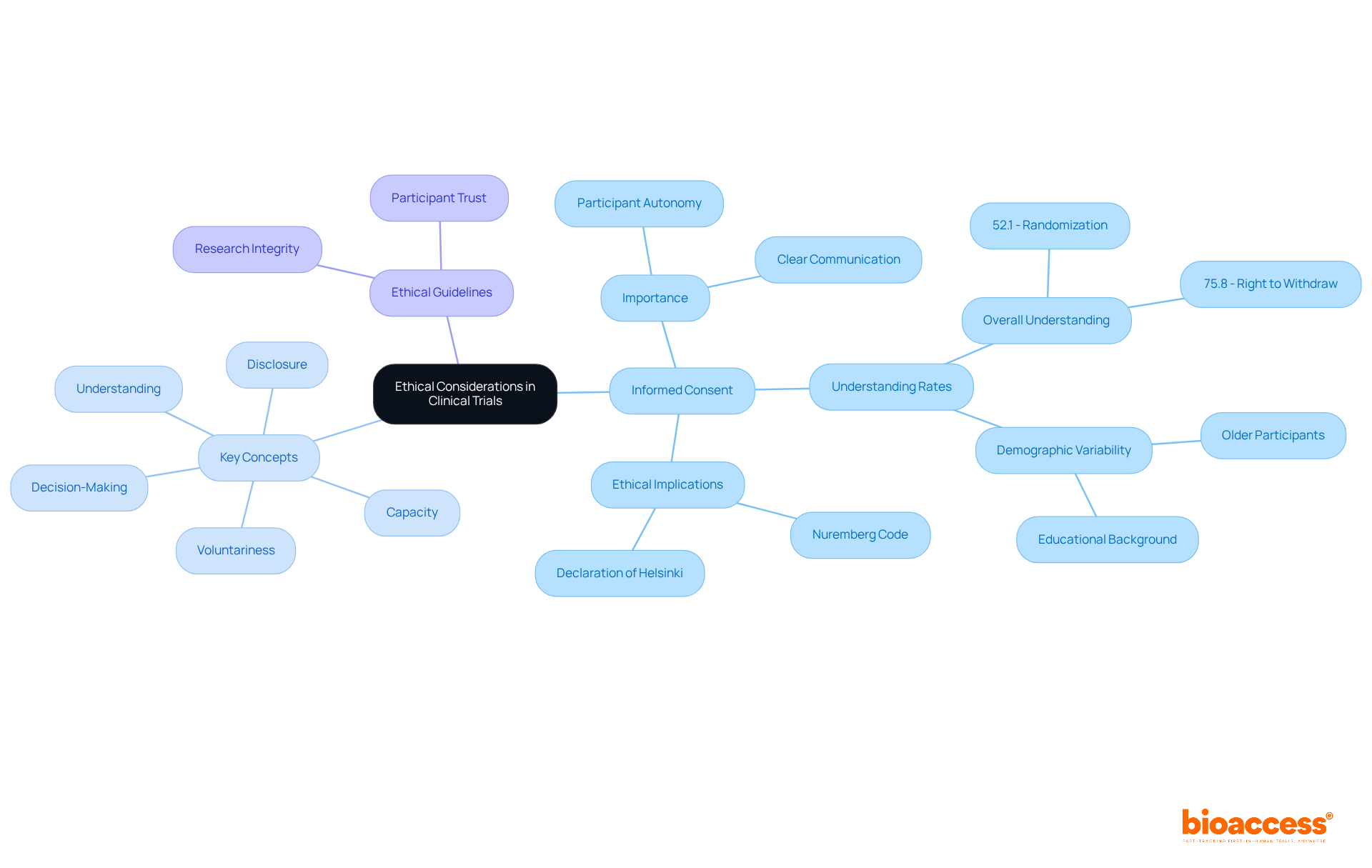
Ethical considerations are fundamental to any clinical trial protocol, with the importance of informed consent being paramount. This process not only honors participant autonomy but also guarantees that individuals are fully informed about the nature of the research, potential risks, and benefits. A systematic review revealed that understanding rates for informed consent components varied from 52.1% to 75.8%, with 75.8% of participants recognizing their right to withdraw from the research at any time, while 67.0% comprehended the potential risks and side effects involved. These statistics underscore the necessity of clear communication during the consent process.
To ensure informed consent, guidelines should outline the processes for obtaining consent, emphasizing five key concepts:
Investigators play a vital role in this, as research suggests that participants who provide consent personally tend to have a better grasp of trial risks compared to those whose guardians sign for them. Moreover, educational interventions can significantly enhance understanding, particularly among participants with lower educational backgrounds.
The clinical trial protocol must also address the ethical implications of research design. Adhering to established ethical guidelines, such as the Nuremberg Code and the Declaration of Helsinki, is essential for maintaining participant trust and research integrity. Additionally, older participants have shown better understanding of the study's nature and withdrawal rights, indicating the importance of considering demographic factors in the consent process. By prioritizing informed consent and participant safety, research studies can uphold ethical standards while advancing medical knowledge.
Involving stakeholders is essential for ensuring that the clinical trial protocol aligns with the requirements and expectations of participants, sponsors, and regulatory agencies. Timely engagement of stakeholders during the development process yields valuable insights into participant preferences and concerns, significantly enhancing the clinical trial protocol for research design and execution.
Effective strategies for stakeholder engagement encompass:
These methods facilitate the collection of feedback that informs procedural adjustments, ultimately leading to improved study feasibility and participant satisfaction. Studies indicate that methods endorsed by key participants are more attractive for recruitment, as they address potential enrollment barriers and elevate the overall quality of the study. By prioritizing stakeholder input, sponsors can create guidelines that are not only scientifically robust but also resonate with the needs of those they aim to serve.
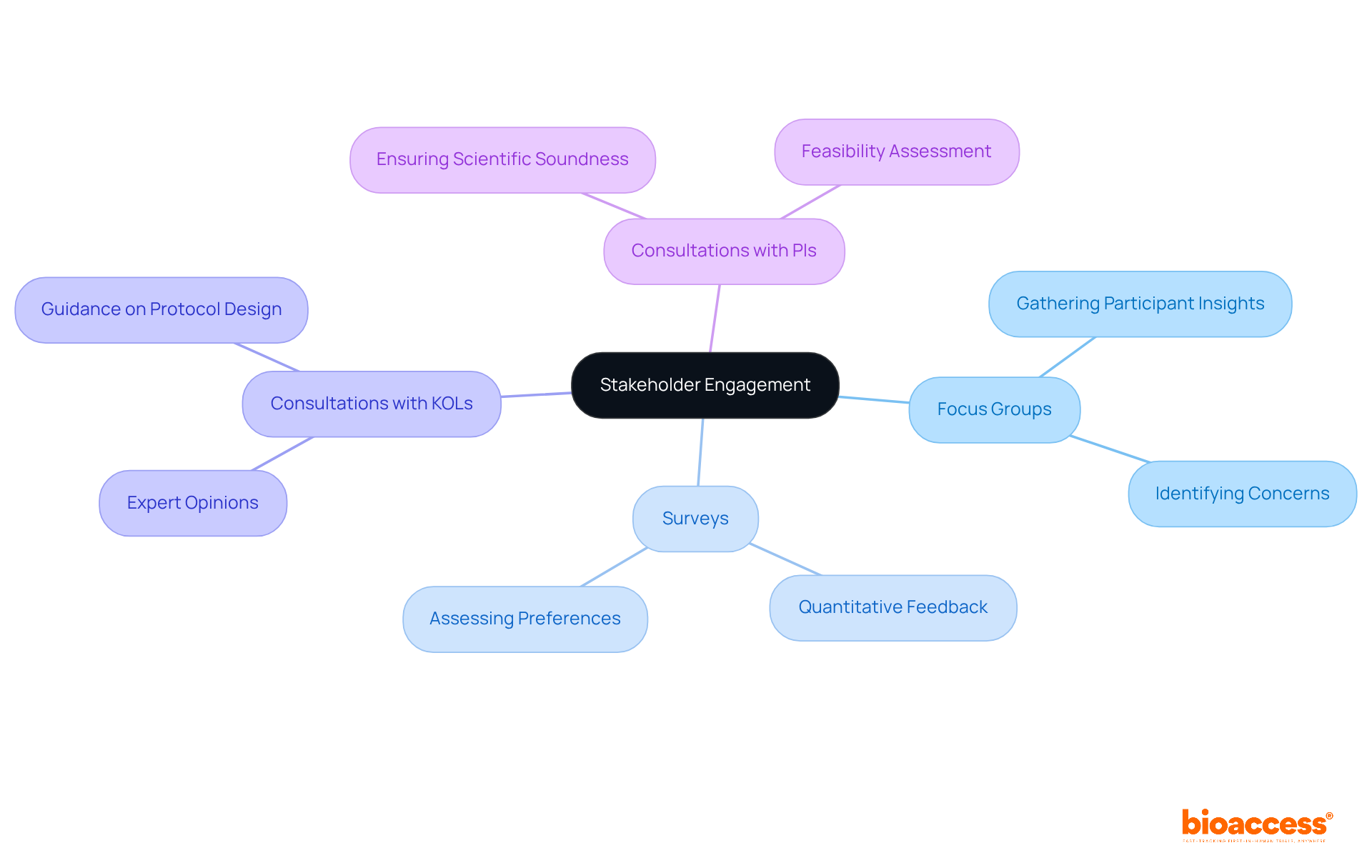
Flexibility in the clinical trial protocol is crucial for effectively overcoming unexpected challenges that may arise during an investigation, particularly for Medtech and Biopharma startups facing recruitment hurdles. This flexibility involves the capability to adjust study designs, recruitment strategies, and data collection methods in response to changing circumstances or new data. For example, the CARISA trial illustrated the significance of modifying recruitment targets based on interim analyses, ultimately improving the trial's efficiency and validity.
To effectively navigate such challenges, researchers must establish clear procedures for modifying guidelines, ensuring all stakeholders are promptly informed of any changes. This proactive strategy not only preserves the integrity of the study but also aligns it with its objectives, even when confronted with unforeseen obstacles. Moreover, data indicates that procedural modifications are a common response to challenges, underscoring the need for a well-structured framework that allows for timely adjustments.
Expert perspectives highlight that a robust protocol should incorporate predefined criteria for modifications, as demonstrated in adaptive designs that facilitate mid-course adjustments based on accumulating results. By fostering a culture of transparency and dialogue, trial participants can adeptly manage the complexities of medical research, ensuring that their investigations remain strong and relevant amidst challenges.
To enhance recruitment efforts, it is vital to leverage comprehensive clinical research management services that adhere to a clinical trial protocol, such as those offered by bioaccess, which include feasibility assessments, site selection, compliance evaluations, and project management. These services are indispensable for tackling recruitment issues and ensuring successful project execution. As a practical measure, researchers should routinely review and update their clinical trial protocol to reflect any shifts in recruitment strategies or study objectives, thereby ensuring alignment with the overarching goals of the trial.

A well-structured clinical trial protocol is indispensable for the successful execution of medical research, acting as a roadmap that directs all stakeholders toward a unified goal. It encompasses critical elements, including:
All of which bolster the integrity and efficacy of the study. By establishing comprehensive guidelines, researchers guarantee that trials are conducted with precision, ultimately facilitating significant advancements in patient care.
Throughout the article, essential components such as:
have been underscored as vital to the protocol's effectiveness. By emphasizing the importance of clarity and adaptability, the discussion highlights the necessity of a well-organized framework that accommodates modifications in response to unforeseen challenges. By addressing common pitfalls and showcasing best practices, the article reinforces the notion that meticulous planning and collaboration are crucial for achieving successful outcomes in clinical trials.
In conclusion, the importance of a comprehensive clinical trial protocol cannot be overstated. It is foundational for ensuring compliance and safeguarding participant welfare while also being instrumental in fostering innovation within the medical field. By prioritizing clarity, ethical standards, and stakeholder engagement, researchers can enhance the quality of clinical trials, ultimately benefiting patients and advancing medical knowledge. Embracing these principles will pave the way for more effective and efficient research endeavors, ensuring that breakthroughs reach those who need them most.
What is the purpose of a clinical research plan?
A clinical research plan serves as a comprehensive document that outlines the objectives, design, methodology, statistical considerations, and operational aspects of a clinical study, acting as a guiding framework to ensure alignment among participants and compliance with regulatory standards.
What key sections are typically included in a comprehensive clinical trial protocol?
Key sections typically include background information, research objectives, participant eligibility criteria, and data collection methods.
Why is it important to include older adults in medical studies?
Including older adults is crucial because they represent a significant demographic in cancer diagnoses, yet only 25 percent of participants in cancer-focused studies are aged 65 and older, highlighting a gap that structured guidelines can help address.
How do research guidelines protect participants and enhance study credibility?
Research guidelines protect participants by incorporating informed consent procedures, safety oversight, and risk management strategies, which also enhance the credibility of the study.
What role does an electronic data capture (EDC) system play in clinical trials?
An EDC system with automated validation checks helps address data entry inconsistencies, thereby safeguarding the integrity of the study.
What are the SMART criteria for research objectives?
The SMART criteria stand for Specific, Measurable, Achievable, Relevant, and Time-bound, which help researchers maintain focus and clarity throughout the research process.
How does a well-crafted research proposal benefit the research process?
A well-crafted research proposal articulates clear objectives that enhance communication and understanding among stakeholders, simplifying research design and ensuring everyone comprehends the intended outcomes.
What is the importance of the protocol review process in clinical research?
The protocol review process is critical for assessing research design, methodology, and ethical considerations, ensuring compliance with clinical trial protocols, Good Clinical Practice (GCP) guidelines, and local regulations.
How does bioaccess® assist in clinical research?
bioaccess® provides comprehensive assistance in managing complexities of clinical trials, including feasibility studies, site selection, compliance reviews, and experiment setup, which enhances the efficiency and effectiveness of studies.