


The landscape of medical device regulation is constantly evolving, making it essential for manufacturers to grasp the intricacies of Post-Market Clinical Follow-up (PMCF). This understanding is crucial for ensuring the safety and efficacy of their products.
In this article, we explore nine key insights that underscore the significance of PMCF in compliance with EU regulations. We will highlight the advantages of streamlined processes and the pivotal role organizations like bioaccess® play in accelerating these activities.
As manufacturers navigate the complexities of regulatory requirements, one pressing question arises: how can they effectively implement PMCF strategies to not only meet compliance but also enhance patient safety and product performance?
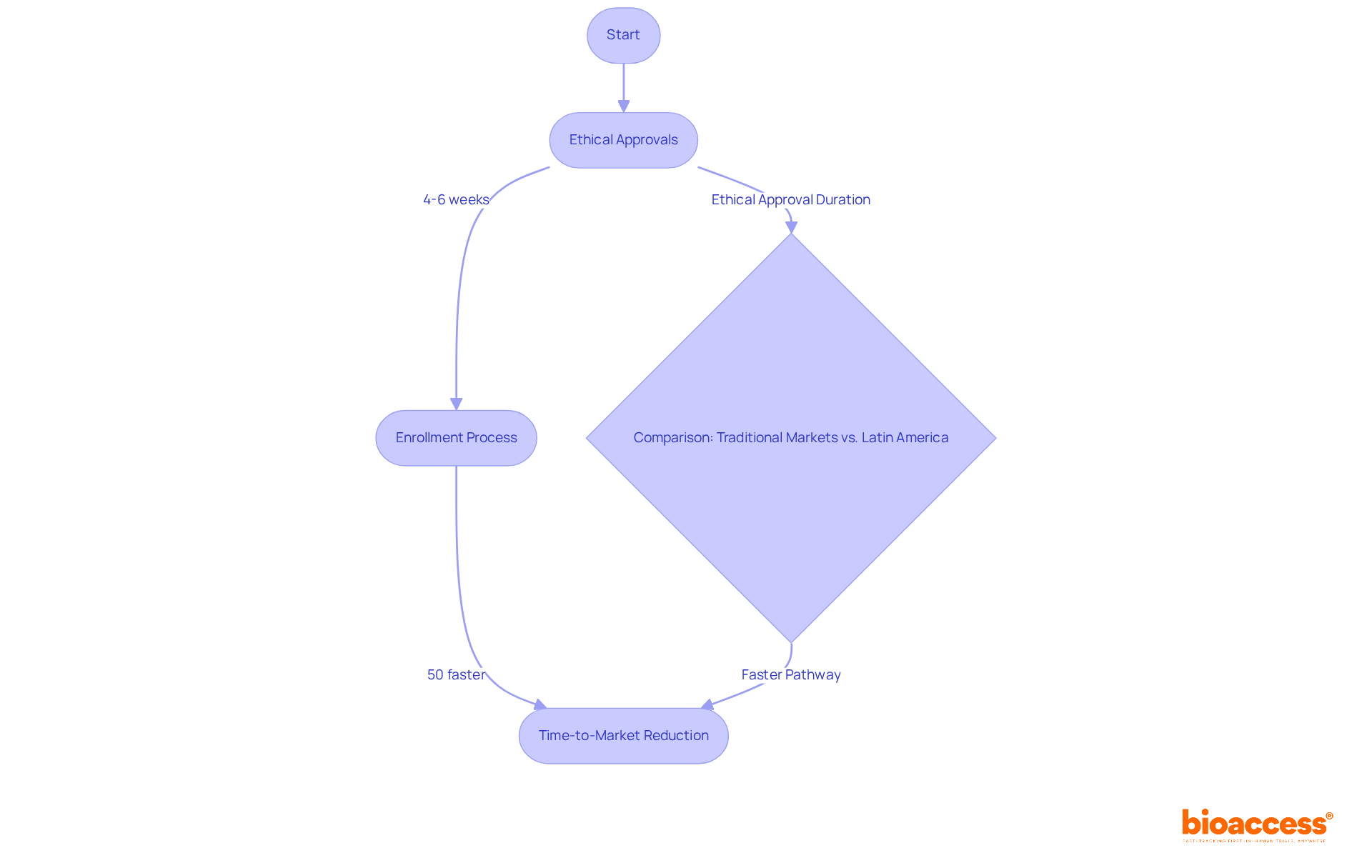
bioaccess® excels in accelerating PMCF medical devices activities for medical instruments, leveraging its deep understanding of regulatory frameworks across Latin America, the Balkans, and Australia. With ethical approvals achieved in an impressive 4-6 weeks and enrollment processes that are 50% faster than traditional markets, bioaccess® provides a streamlined pathway that significantly reduces time-to-market for medical devices. This rapid agility is essential for manufacturers aiming to maintain compliance while ensuring the safety and effectiveness of their products in real-world applications.
Current trends indicate that the typical duration for ethical approvals in Latin America is notably shorter than the 18 months often seen in the EU, making this region an increasingly attractive option for PMCF medical devices activities. Successful implementations of these follow-ups in Latin America have demonstrated substantial cost savings and quicker timelines, reinforcing the strategic advantages of conducting clinical trials in this vibrant environment. Industry leaders stress that regulatory speed is crucial for sustaining a competitive edge, and bioaccess® stands at the forefront of facilitating this vital process.
Post-Market Clinical Follow-up (PMCF) and Post-Market Performance Follow-up (PMCF) medical devices are critical components of the EU Medical Device Regulation and In Vitro Diagnostic Regulation. These processes are designed to systematically gather clinical information regarding the safety and effectiveness of medical instruments after they hit the market, while another method focuses on the performance of in vitro diagnostic instruments. Compliance with these procedures is not optional; manufacturers must create detailed plans that articulate their methods, objectives, and timelines for data collection and analysis.
Recent statistics reveal that a significant number of medical device manufacturers struggle with full compliance regarding PMCF medical devices and performance follow-up regulations. This highlights the urgent need for meticulous planning and execution. Regulatory experts emphasize that a well-structured post-market clinical follow-up strategy should clearly define the clinical questions it aims to address, ensuring that the data collected effectively bridges any gaps in clinical assessment.
The importance of post-market clinical follow-up and PMCF medical devices cannot be overstated, as they are vital for ongoing safety evaluations and adherence to regulations. These processes enable manufacturers to identify potential risks early, validate benefit-risk assessments, and ensure that PMCF medical devices remain safe and effective throughout their lifecycle. Furthermore, recent changes in PMPF regulations in Europe underscore the necessity for continuous monitoring and proactive data collection, making it imperative for manufacturers to stay informed and adaptable.
Examples of manufacturers successfully developing post-market clinical follow-up plans illustrate the practical application of these requirements. By integrating insights from regulatory guidance and employing methodologies such as literature reviews and patient surveys, these companies not only meet regulatory demands but also enhance their product development processes. This proactive approach builds trust among stakeholders and contributes to the overall advancement of medical technology.
Post-market clinical follow-up and performance follow-up are vital for ensuring the ongoing safety and efficacy of medical instruments, whether they are newly introduced or legacy products. For new products, post-market clinical follow-up provides essential information that verifies safety and performance in real-world settings. In the case of legacy equipment, it addresses any clinical data gaps that may have emerged since the product's initial approval. This continuous evaluation is crucial for maintaining compliance with regulatory standards and safeguarding patient safety.
Recent research highlights that effective implementation of post-market clinical follow-up can significantly enhance our understanding of legacy equipment's performance, allowing for the swift identification and resolution of emerging risks. Industry leaders emphasize that a robust post-market clinical follow-up strategy is essential for manufacturers to uphold high compliance standards and foster trust with regulatory bodies. Specific activities, such as controlled trials with randomized designs, are critical for thoroughly assessing the efficacy and safety of medical devices.
Moreover, manufacturers must integrate post-market clinical follow-up results into their Clinical Evaluation Reports (CER), demonstrating their commitment to regulatory adherence. Successful examples of post-market clinical follow-up for legacy devices include targeted data collection through registry analyses and feedback mechanisms from healthcare professionals and patients, which yield invaluable insights into device efficacy and safety.
At bioaccess®, we leverage over 20 years of Medtech expertise to efficiently manage post-market clinical follow-up studies. Our extensive services, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), ensure that our clients navigate the complexities of clinical trials with confidence. Collaboration is key, and we invite you to partner with us to enhance your clinical research efforts.
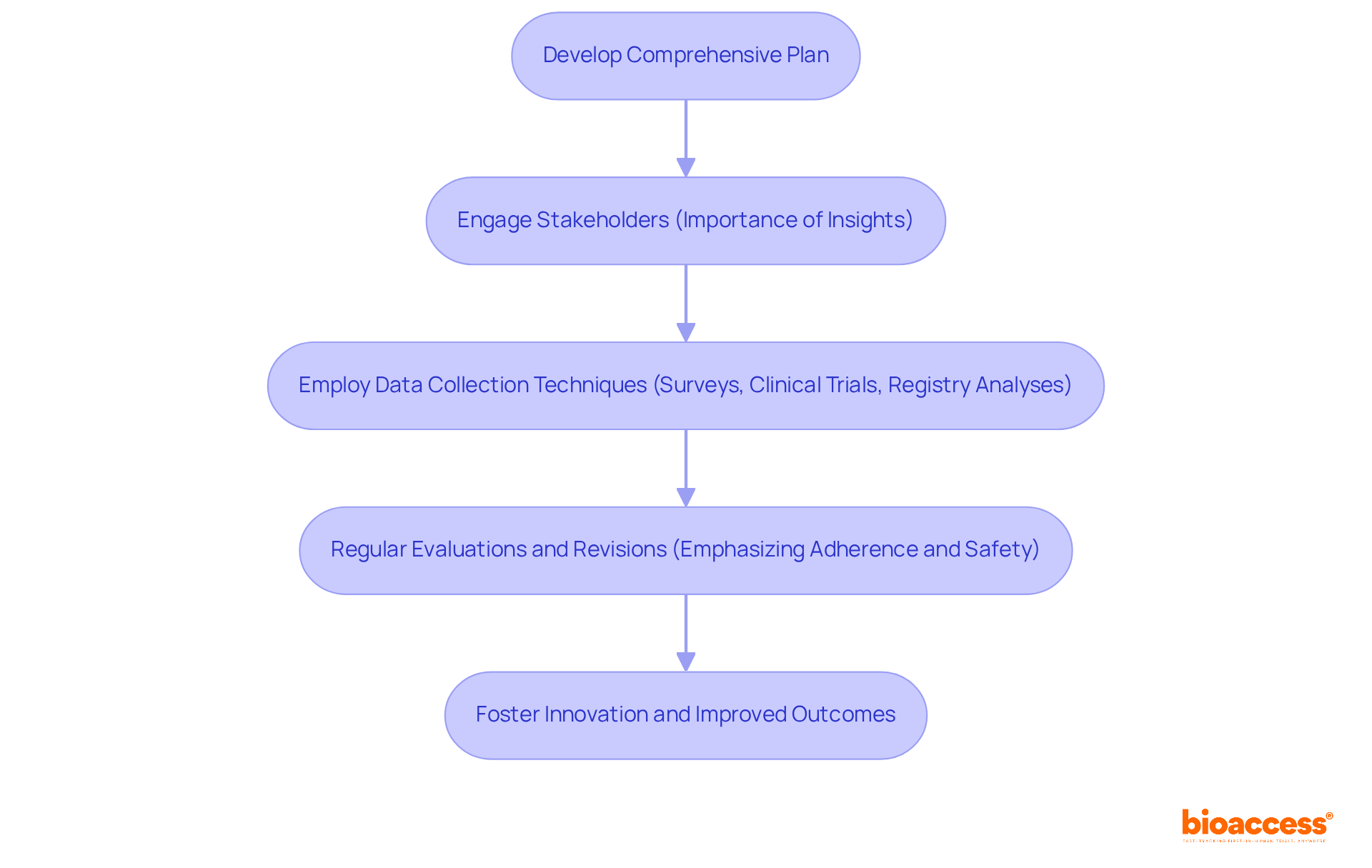
To execute effective strategies for PMCF medical devices and Post-Market Performance Follow-up, manufacturers must develop a comprehensive plan detailing specific objectives, methodologies, and timelines. Engaging stakeholders - such as healthcare professionals and patients - provides critical insights into the real-world application of the device, enhancing its relevance and effectiveness. Recent research indicates that a comprehensive initiative significantly boosts stakeholder involvement, with 30% of participants noting improved representation and engagement processes.
Employing a mix of data collection techniques, including surveys, clinical trials, and registry analyses, enhances the reliability of the collected data. Regular evaluations and revisions of the PMCF medical devices follow-up strategy, in response to new discoveries, are crucial for ensuring adherence and addressing emerging safety issues. This proactive approach not only meets regulatory requirements but also fosters ongoing innovation and improved patient outcomes.
At bioaccess®, our over 20 years of expertise in overseeing post-market clinical follow-up studies in Latin America ensures that we deliver thorough clinical trial management services. From feasibility assessments to project oversight and reporting, we customize our services to address the distinct requirements of each product and its market context. Collaboration is key - let's work together to navigate the complexities of clinical research and drive better outcomes.
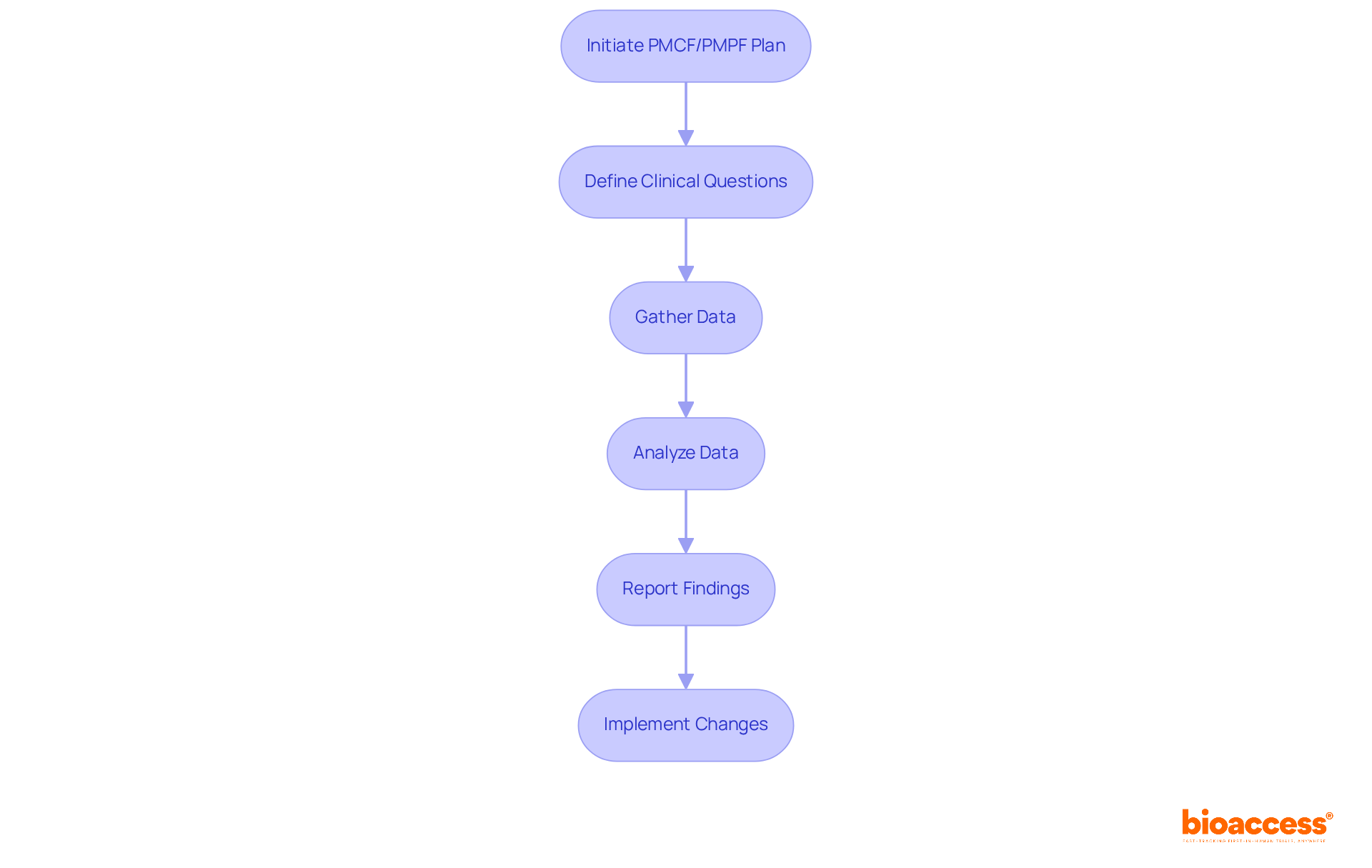
Key activities in Post-Market Clinical Monitoring and Post-Market Performance Follow-Up processes are essential for ensuring the ongoing safety and effectiveness of medical devices. A well-structured PMCF plan lays the groundwork, clearly outlining objectives, methodologies, and timelines for information collection. Various methods, such as surveys, clinical trials, and user feedback mechanisms, are employed to gather real-world evidence. Regulatory experts stress that effective data collection must be intentional; as Mark Twain wisely noted, "data without a clear plan is rendered useless."
Data analysis is crucial for interpreting the information collected. Manufacturers must apply advanced statistical techniques to evaluate device performance and identify any adverse events. This process includes thorough documentation of results to regulatory authorities, ensuring compliance with the EU Medical Device Regulation (MDR), which mandates routine post-market clinical follow-up activities as part of a comprehensive post-market surveillance system. For example, analyzing adverse event reports is vital, as it enables manufacturers to address emerging safety concerns and refine their risk management strategies.
Recent advancements in post-market clinical follow-up data collection methods, including the integration of digital tools and real-time monitoring systems, significantly enhance the efficiency and accuracy of data gathering. Engaging with users and stakeholders throughout the process is critical, as their insights can inform future actions and bolster product safety. This collaborative approach not only fosters transparency but also improves the overall efficiency of post-market clinical follow-up and surveillance processes, ensuring that medical devices consistently meet regulatory standards and provide value to patients. Furthermore, for manufacturers outside the European Union, the post-market clinical follow-up plan must include the name and address of the authorized representative, reinforcing compliance and accountability. At bioaccess®, we bring over 20 years of Medtech expertise to tackle these challenges, ensuring that your post-market clinical follow-up studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and others, are executed with the utmost proficiency and tailored to meet regulatory standards in Latin America.

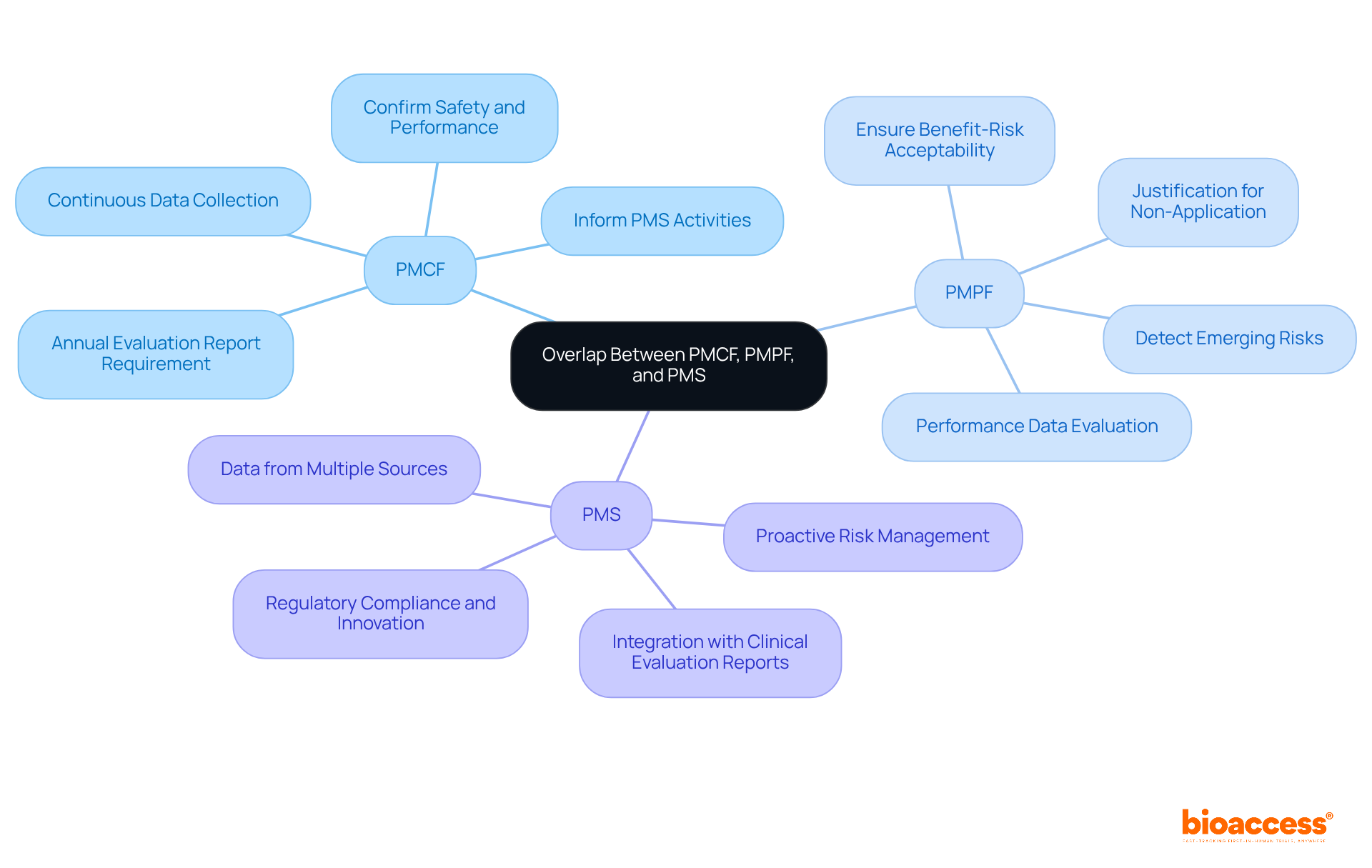
PMCF medical devices and Post-Market Surveillance (PMS) are crucial components in ensuring the ongoing safety and efficacy of medical devices. By focusing on the continuous collection of clinical information and evaluating performance metrics, these processes guarantee the safety, effectiveness, and scientific credibility of PMCF medical devices throughout their expected lifespan. Understanding the overlap between PMPF and PMS allows manufacturers to strengthen their compliance efforts. Insights gained from PMCF medical devices can significantly inform post-market surveillance activities and vice versa. This integrated approach not only streamlines regulatory compliance for PMCF medical devices but also enhances the overall effectiveness of post-market monitoring.
For instance, information gathered through PMCF medical devices can reveal new risks and performance patterns, which are essential for post-market surveillance activities aimed at identifying the need for corrective measures. Industry leaders emphasize that a well-organized PMS system, which incorporates insights on PMCF medical devices, can transform compliance from a mere obligation into a strategic asset that fosters innovation and builds trust among stakeholders. As David Rutledge, President & CEO at Global Strategic Solutions, aptly states, "PMS should be an active system that detects patterns early, captures new risks, and identifies opportunities."
Moreover, with the impending deadlines for EU MDR and IVDR compliance-including the requirement for a PMS report for Class I products and the annual update of the PSUR for Class IIb and Class III products-the urgency of adopting an integrated approach to post-market monitoring of PMCF medical devices becomes increasingly evident. By leveraging the synergies between PMCF medical devices and PMS, manufacturers can take a proactive approach in monitoring device performance, ultimately enhancing patient safety and product efficacy. To effectively implement this integrated approach, manufacturers should regularly assess and update their post-market clinical follow-up and post-market surveillance plans to align with evolving regulatory requirements and emerging clinical information.
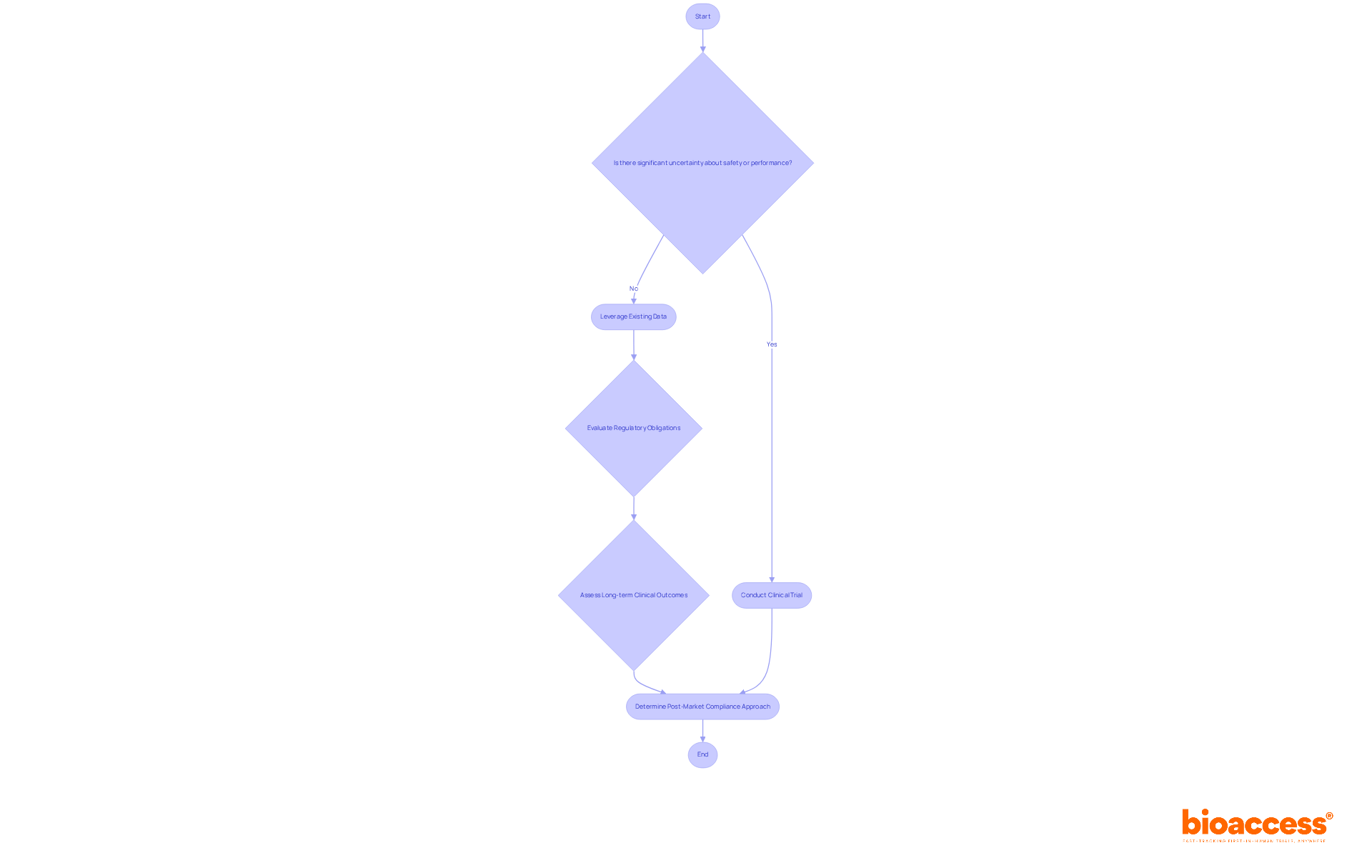
Clinical studies are not universally mandated for Post-Market Clinical Follow-Up and Post-Market Performance Follow-Up. Producers often leverage existing clinical information, literature assessments, and real-world evidence from registries and surveys to meet their post-market responsibilities. For example, proactive screening of relevant scientific literature can effectively align with both Post-Market Surveillance (PMS) and post-market clinical follow-up activities, thereby streamlining compliance processes.
However, if significant uncertainties regarding a product's safety or performance persist, conducting a clinical trial may become essential to gather the necessary data. Regulatory guidelines emphasize that the decision to undertake post-market clinical follow-up assessments should be based on identifying potential remaining risks and the clarity of long-term clinical outcomes, which directly impacts the benefit-risk balance of the medical device. Therefore, producers must carefully evaluate their specific circumstances and regulatory obligations to determine the most appropriate approach for post-market clinical follow-up compliance.
Pre-market research primarily focuses on gathering essential information to facilitate the initial authorization of medical instruments, often conducted in regulated environments with specific patient groups. In contrast, post-market evaluations, such as Post-Market Clinical Follow-up and Post-Market Performance Follow-up, play a vital role in monitoring the ongoing safety and performance of devices once they reach the market. These investigations aim to collect data from a broader, real-world user base, providing insights crucial for manufacturers to effectively navigate regulatory requirements.
bioaccess® excels in comprehensive clinical trial management services, including:
Drawing on over 20 years of expertise in the Medtech sector, our approach encompasses:
This ensures that each evaluation is executed efficiently and effectively.
Recent findings indicate that nearly 79% of manufacturers conducting post-market assessments do so to monitor product performance and safety, underscoring the effectiveness of these evaluations in identifying potential risks. Industry leaders stress the importance of these analyses, with one stating, "your most dissatisfied customers are your greatest source of learning," highlighting the value of feedback in enhancing product safety. Additionally, the FDA has set forth requirements for ongoing post-market surveillance, including mandatory reporting of adverse events, which reinforces the necessity of robust post-market studies in ensuring device safety and efficacy.

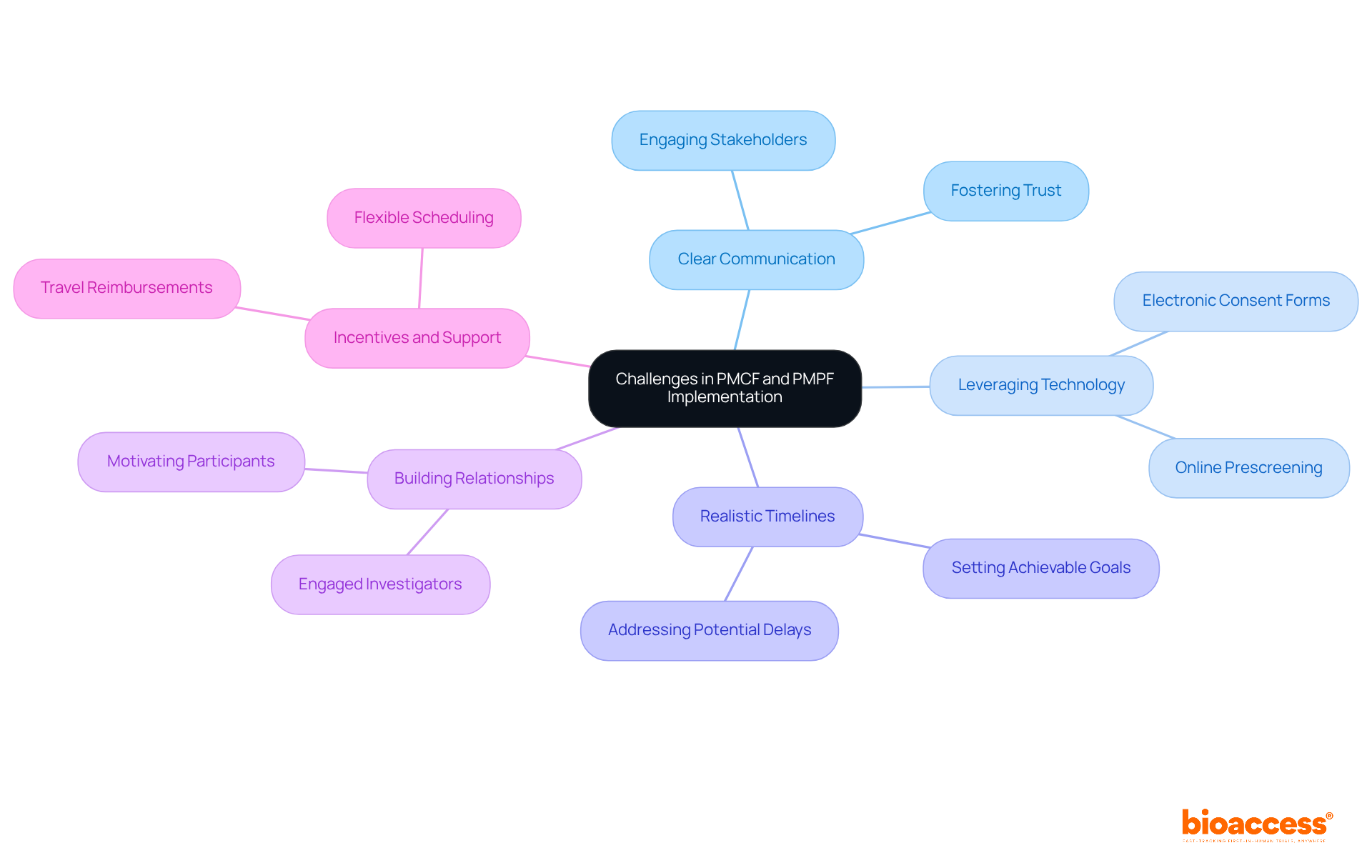
Executing Post-Market Clinical Follow-up and Post-Market Surveillance presents significant challenges, particularly in patient recruitment, information gathering, and regulatory adherence. These hurdles demand strategic approaches from manufacturers to ensure effective navigation.
Clear Communication is vital. Establishing transparent communication strategies engages stakeholders - patients, clinical sites, and investigators - fostering trust and encouraging participation.
Leveraging Technology can streamline data collection processes, enhancing efficiency and reducing participant burden. Tools like electronic consent forms and online prescreening facilitate smoother recruitment.
Realistic Timelines are crucial. Setting achievable timelines that consider potential delays is essential, as recruitment difficulties can lead to significant setbacks, costing the pharmaceutical industry between $600,000 and $8 million for each day of delay.
Building Relationships with clinical sites and investigators enhances recruitment efforts. Engaged investigators are more likely to motivate potential participants and effectively address their concerns.
Incentives and Support play a key role. Offering incentives, such as travel reimbursements and flexible scheduling, alleviates participant burdens and improves recruitment rates. Research indicates that financial support significantly boosts patient recruitment success.
Data reveals that 37% of clinical trial sites under-enroll volunteers, underscoring the necessity for proactive recruitment strategies. Successful post-market clinical follow-up studies for pmcf medical devices often incorporate dynamic recruitment strategies tailored to the specific context of each trial, addressing participant motivations and obstacles. By focusing on these strategies, manufacturers can enhance recruitment outcomes and ensure the success of post-market clinical follow-up and performance framework activities.
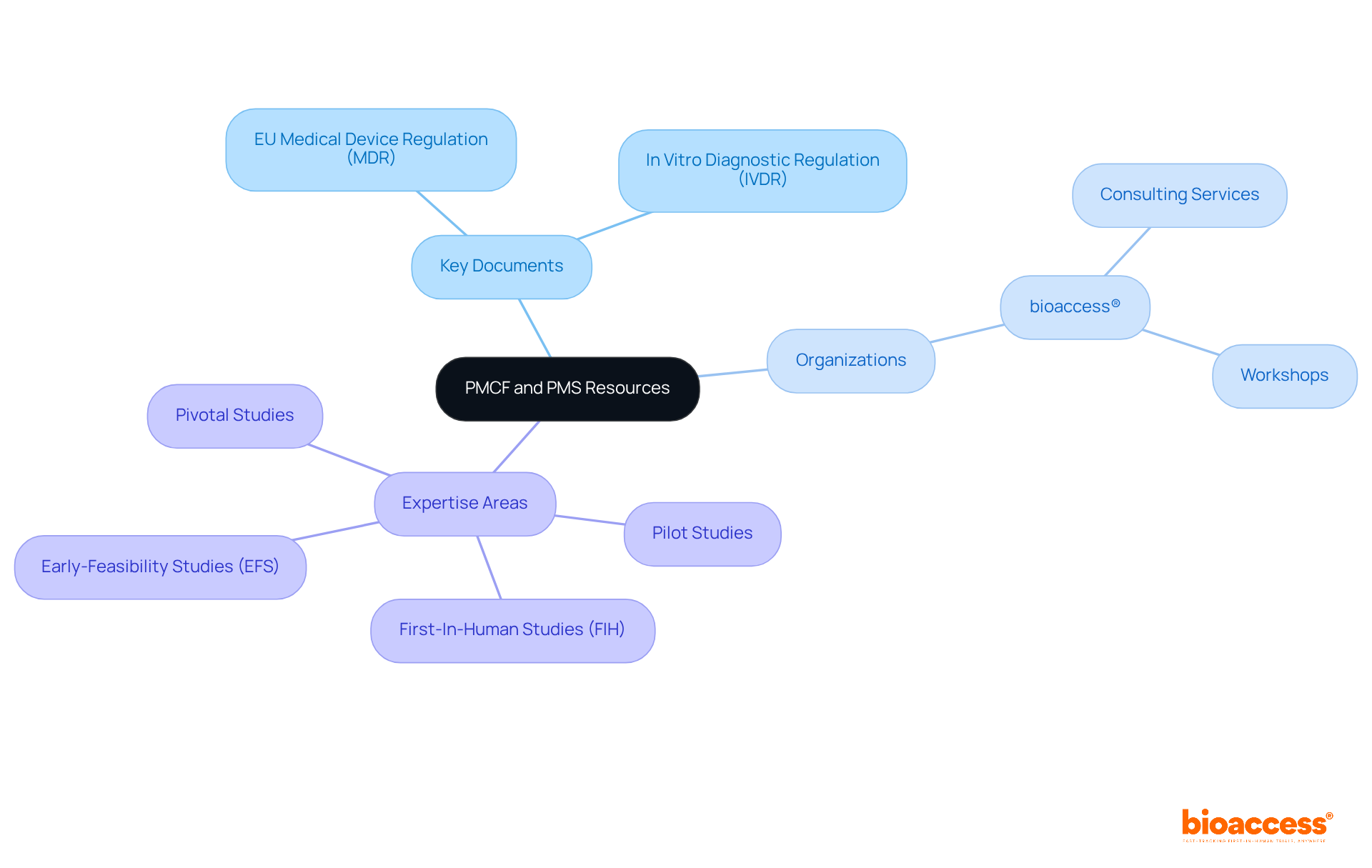
To enhance your understanding of Post-Market Clinical Follow-up (PMCF) and Post-Market Surveillance (PMS), a wealth of resources is at your disposal. Key documents such as the EU Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) are essential, along with guidance from the Medical Device Coordination Group (MDCG), which offers critical insights into compliance requirements. Have you considered how industry publications can illuminate best practices and case studies? They provide valuable insights into effective post-market clinical follow-up strategies.
Organizations like bioaccess® play a pivotal role in this landscape, offering specialized consulting services and workshops designed to help manufacturers navigate the complexities of PMCF medical devices and PMS. This support ensures that you remain compliant and competitive in the ever-evolving medical device environment. With a tailored strategy that addresses your unique needs, bioaccess® leverages its expertise in:
to guide you through the regulatory landscape.
To maximize your efforts in PMCF medical devices, consider implementing a robust compliance framework. Staying updated on the latest methodologies discussed in industry journals is crucial. Collaboration is key - by working together, we can tackle the challenges of clinical research head-on. What steps will you take next to enhance your PMCF strategy?
The significance of Post-Market Clinical Follow-up (PMCF) and Post-Market Performance Follow-up (PMPF) for medical devices is paramount. These processes are essential for ensuring the ongoing safety and effectiveness of medical instruments in real-world settings. Manufacturers must navigate complex regulatory landscapes, making it crucial to understand the nuances of PMCF and PMPF for maintaining compliance and enhancing product reliability.
This article has provided key insights into how manufacturers can optimize their PMCF and PMPF strategies. From the benefits of conducting clinical trials in regions like Latin America to the necessity of integrating stakeholder feedback, the discussion underscores the critical role that effective planning and execution play in achieving regulatory success. Furthermore, the overlap between PMCF, PMPF, and Post-Market Surveillance (PMS) highlights the need for a cohesive approach to monitoring device performance and safety.
As the medical device industry evolves, staying informed about the latest trends and regulatory requirements is vital. Manufacturers are encouraged to adopt proactive strategies that not only meet compliance obligations but also foster innovation and enhance patient safety. Engaging with resources, leveraging technology, and promoting collaboration will be essential steps in overcoming challenges and improving the effectiveness of PMCF and PMPF activities. By prioritizing these efforts, stakeholders can ensure that medical devices not only comply with regulations but also deliver significant value to patients and healthcare systems alike.
What is bioaccess® and what services does it provide for PMCF activities?
bioaccess® specializes in accelerating Post-Market Clinical Follow-up (PMCF) activities for medical devices, leveraging its understanding of regulatory frameworks in regions like Latin America, the Balkans, and Australia. It achieves ethical approvals in 4-6 weeks and speeds up enrollment processes by 50% compared to traditional markets, helping manufacturers reduce time-to-market while ensuring compliance and product safety.
How does the duration of ethical approvals in Latin America compare to the EU?
The typical duration for ethical approvals in Latin America is significantly shorter than the 18 months often required in the EU, making Latin America an attractive option for conducting PMCF activities for medical devices.
What are PMCF and PMPF, and why are they important?
Post-Market Clinical Follow-up (PMCF) and Post-Market Performance Follow-up (PMPF) are processes mandated by the EU Medical Device Regulation and In Vitro Diagnostic Regulation to gather clinical information on the safety and effectiveness of medical devices after they are on the market. They are essential for ongoing safety evaluations, risk identification, and ensuring that devices remain compliant with regulatory standards.
What challenges do manufacturers face regarding PMCF compliance?
Many medical device manufacturers struggle with full compliance concerning PMCF and PMPF regulations, highlighting the need for meticulous planning and execution of their post-market strategies.
What should a well-structured PMCF strategy include?
A well-structured PMCF strategy should clearly define the clinical questions it aims to address, outline methods, objectives, and timelines for data collection and analysis, ensuring that the data effectively bridges any gaps in clinical assessments.
How do PMCF activities benefit both new and legacy medical devices?
For new products, PMCF provides essential information verifying safety and performance in real-world settings. For legacy products, it addresses clinical data gaps that may have arisen since initial approval, ensuring ongoing compliance and patient safety.
What are some effective methods for implementing PMCF?
Effective PMCF implementation can include controlled trials with randomized designs, registry analyses, and feedback mechanisms from healthcare professionals and patients, which provide valuable insights into device efficacy and safety.
How does bioaccess® support post-market clinical follow-up studies?
bioaccess® utilizes over 20 years of Medtech expertise to efficiently manage post-market clinical follow-up studies, offering services such as Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH) to help clients navigate clinical trial complexities.