


This article highlights the essential regulations that medical devices must meet for successful market access, with a particular emphasis on the Medical Device Regulation (MDR), In Vitro Diagnostic Regulation (IVDR), and other compliance standards. Adhering to these regulations, including obtaining CE marking and implementing quality management systems such as ISO 13485, is crucial. Such compliance not only ensures patient safety but also enhances product reliability and facilitates faster entry into global markets.
The landscape of medical device regulation is increasingly complex, presenting manufacturers with a myriad of requirements that are essential for compliance and successful market access. As the healthcare sector evolves, grasping the key regulations—such as the Medical Device Regulation (MDR), In Vitro Diagnostic Regulation (IVDR), and ISO standards—becomes critical for companies seeking to navigate this intricate environment. With the stakes higher than ever, how can manufacturers effectively position themselves to not only meet these stringent standards but also accelerate their entry into diverse global markets? This article delves into nine essential regulations shaping the future of medical device compliance, offering insights and strategies to empower industry players in their quest for success.
bioaccess® leverages its extensive knowledge of regulation medical devices to empower Medtech and Biopharma firms in achieving compliance with health product regulations. By harnessing the regulatory efficiency of Latin America, where ethical approvals can be secured in just 4-6 weeks, and tapping into the diverse patient populations in the Balkans alongside streamlined pathways in Australia, bioaccess® adeptly navigates the intricate landscape of compliance. This strategic approach not only accelerates the approval process but also enhances the quality of clinical research, facilitating faster market access for innovative health products.
With the demand for regulatory affairs services on the rise—projected to reach USD 30.16 billion by 2030, expanding at a CAGR of 9.1%—bioaccess® establishes itself as a frontrunner in this dynamic environment, ensuring that clients can swiftly adapt to evolving regulations and seize emerging opportunities in the healthcare market.
The regulation medical devices, specifically the Medical Equipment Regulation (MDR), establishes stringent requirements for medical instruments aiming for entry into the European market. Central to these requirements is the necessity for comprehensive technical documentation, detailing the design, manufacturing processes, and intended use of the devices. Additionally, manufacturers must implement robust risk management procedures that identify, assess, and mitigate potential hazards associated with their products. Post-market surveillance strategies are equally critical, ensuring ongoing oversight of product performance and safety following market entry.
Adhering to the MDR is essential for obtaining CE marking, a certification that signifies compliance with EU safety standards. This marking serves not only as a legal obligation but also as a pivotal element in fostering market trust and acceptance. Regulatory experts emphasize that compliance with the regulation medical devices, particularly the MDR, represents a continuous commitment to patient safety and product quality, highlighting the importance of integrating these adherence measures into the overall business strategy. As one prominent regulatory authority articulated, "Regulatory compliance is not just a hurdle to be cleared; it’s an ongoing commitment to patient safety and product quality."
Recent data indicates that as of May 2024, the number of healthcare instruments successfully securing CE marking under the MDR has been significantly impacted by the stringent criteria, with numerous manufacturers facing challenges in meeting the new standards. The validation period for a healthcare instrument under the MDR has now extended to 13-18 months, underscoring the prolonged certification timeline that producers must navigate. Furthermore, 81% of respondents report finding the MDR challenging, reflecting a prevailing sentiment within the industry regarding the complexity of the regulation. The regulation medical devices, specifically the MDR's emphasis on essential safety and performance standards, aims to enhance patient safety and reduce the likelihood of product recalls, ultimately fostering a more reliable medical market within the EU. Additionally, 89% of surveyed companies express a preference for prioritizing the United States over the European Union, highlighting the challenges and perceptions surrounding the EU market in comparison to the US.
The In Vitro Diagnostic Regulation (IVDR) establishes essential compliance standards for producers of diagnostic instruments, underscoring its relevance in the clinical research landscape. Central to these requirements is the performance evaluation plan, which must convincingly demonstrate the instrument's clinical performance and safety. This plan is bolstered by robust clinical evidence, reflecting a significant increase in applications for clinical performance studies—approximately 25-30%—following the IVDR's implementation. Furthermore, producers are mandated to establish a quality management system (QMS) in accordance with ISO 13485 standards, ensuring thorough supervision throughout the product lifecycle.
A pivotal aspect of IVDR compliance is the regulation of medical devices based on risk categorization, which dictates the level of scrutiny during the approval process. Under the new regulation medical devices, the proportion of in vitro diagnostics requiring notified body involvement has surged from about 20% to 80-90%. This dramatic shift highlights the imperative for producers to actively collaborate with notified bodies to adeptly navigate the complexities of the new classification system. The IVDR categorizes devices into four classes (A, B, C, and D), with increasing levels of scrutiny reflecting their associated risks.
Industry leaders emphasize the critical role of clinical evidence in this regulatory framework. As Wade Schroeder articulates, "the IVDR introduces a risk-based classification system that requires thorough risk analysis for IVD producers, which is essential for the regulation of medical devices." This approach not only enhances patient safety but also facilitates market access for innovative diagnostic solutions. Notably, about 40% of initial protocol submissions necessitate substantial revisions following regulatory or ethics committee review, underscoring the challenges producers face in the adherence process. Additionally, the IVDR emphasizes improved post-market monitoring and vigilance reporting obligations, making compliance essential for producers striving to maintain their competitive edge in the EU market.
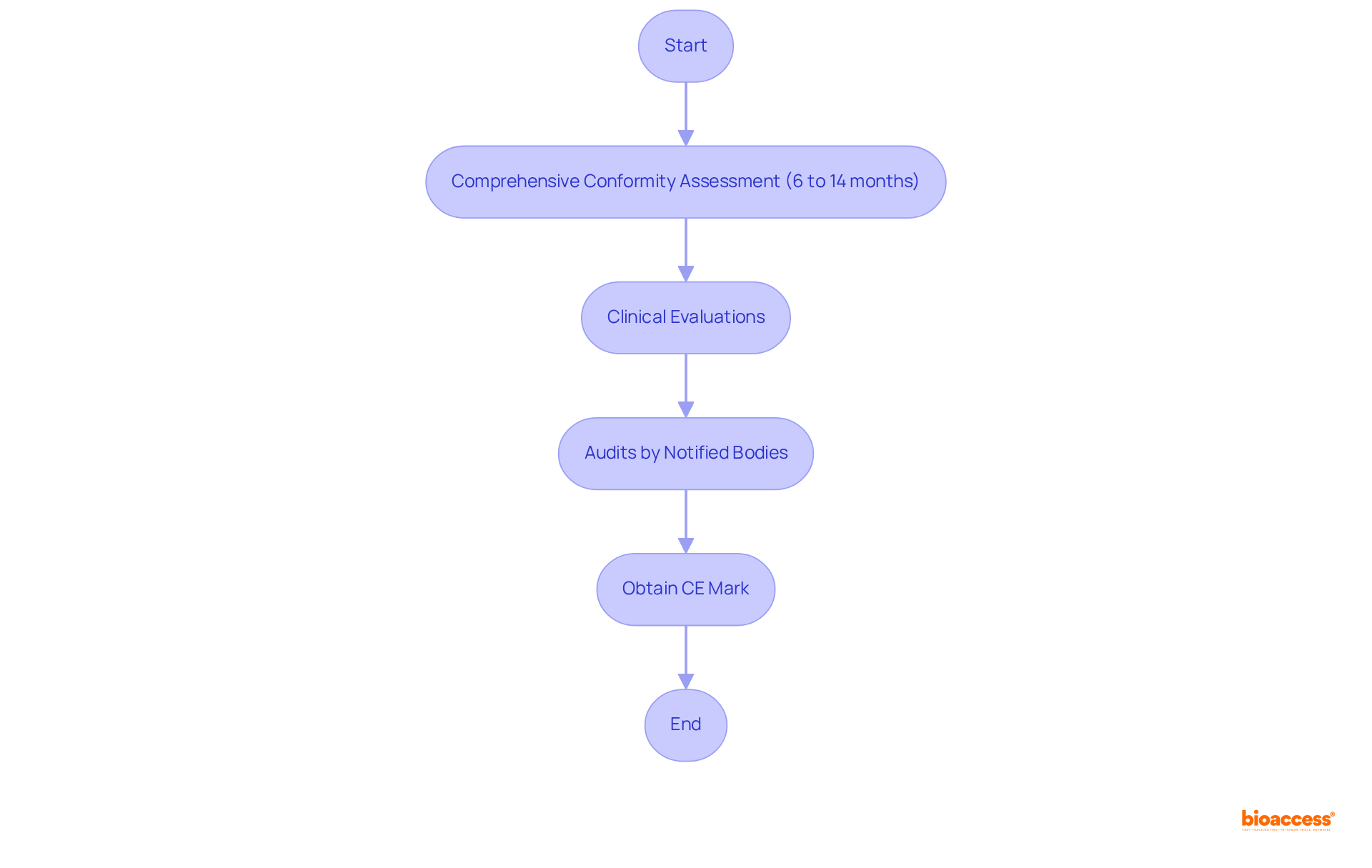
CE marking represents a vital certification for health-related products intended for sale in the European Economic Area (EEA), signifying compliance with rigorous EU safety and performance standards. The process of obtaining CE marking necessitates a comprehensive conformity assessment, which may encompass clinical evaluations and audits conducted by Notified Bodies. Manufacturers should anticipate a timeframe of 6 to 14 months for this evaluation, contingent upon the product's complexity and the Notified Body's workload. However, manufacturers can manage CE marking activities within a more streamlined 2-4 months, offering a clearer perspective on the timeline options available.
The importance of CE marking is paramount, as it serves as a legal prerequisite for market entry in Europe. Products that achieve CE marking not only affirm their compliance with essential regulations but also bolster their credibility in the marketplace. Notably, research indicates that:
This stark contrast underscores the rigorous assessment process associated with CE marking.
Recent updates to the conformity assessment process reflect ongoing changes in regulation medical devices, including the introduction of the EU Artificial Intelligence Act. This act significantly impacts the approval of AI/ML-based healthcare products by establishing specific requirements for their safety and efficacy. Manufacturers are urged to collaborate closely with regulatory experts to adeptly navigate the evolving regulation medical devices requirements. By ensuring well-prepared technical documentation and compliance with General Safety and Performance Requirements (GSPR), companies can expedite their CE marking process, ultimately facilitating successful market access in Europe.
ISO 13485 stands as a pivotal global standard delineating the criteria for a quality management system (QMS) tailored specifically for the healthcare product sector. Adhering to ISO 13485 is imperative for producers, as it ensures the consistent creation of safe and effective healthcare products. The standard emphasizes risk management, mandating organizations to embrace a risk-based approach to quality assurance. This focus not only aids in identifying and mitigating potential risks but also cultivates a culture of continuous improvement within the organization.
The importance of adhering to ISO 13485 is underscored by its role in enhancing customer satisfaction and meeting regulatory requirements. Organizations that implement ISO 13485 frequently experience substantial benefits, including improved operational efficiency and diminished risk exposure. For example, companies that embrace ISO 13485 standards can realize savings of at least $25,000 within the first year of implementation, underscoring the financial advantages of upholding high-quality standards. Additionally, the cost of poor quality can range from 15 to 35 percent of total business costs in regulated industries, further accentuating the necessity for effective quality management.
Quality management specialists assert that achieving ISO 13485 compliance transcends mere legal obligations; it represents a commitment to excellence and safety in the manufacturing of healthcare products. As industry leaders note, integrating a robust QMS aligned with ISO 13485 can yield a 30% increase in the identification of safety issues through effective post-market surveillance (PMS). This proactive approach not only enhances product reliability but also shields organizations from potential legal and regulatory challenges. Notably, nearly 70% of healthcare equipment firms lack the capabilities required to comply with regulation medical devices, which emphasizes the critical need for staff training on QMS guidelines.
In conclusion, ISO 13485 serves as a foundational framework for quality management in the medical device industry, steering organizations toward operational excellence while ensuring compliance with stringent regulation medical devices. The timeline for achieving ISO 13485 certification typically ranges from 3-6 months for smaller entities to as long as 12 months for larger organizations, reflecting the commitment necessary to meet these standards. Furthermore, entities such as INVIMA play a crucial role in advancing the PMS process, ensuring alignment with international standards and bolstering regulatory compliance in the Medtech industry.
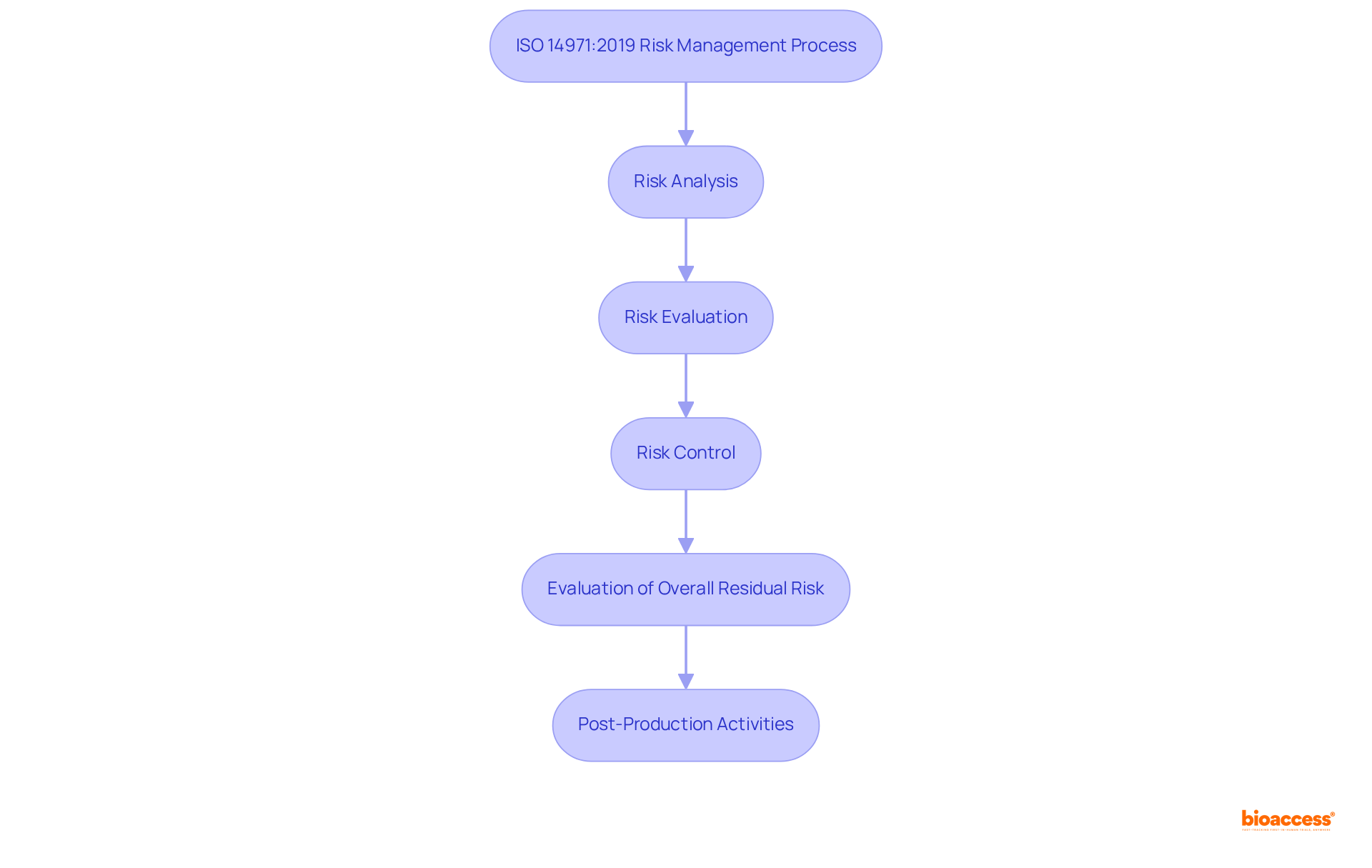
ISO 14971:2019 establishes a critical framework for medical device creators to systematically identify, evaluate, and mitigate risks throughout the product lifecycle, from initial design to post-market activities. This standard mandates a structured approach to risk management, which is essential for enhancing patient safety and ensuring compliance with regulation medical devices. By adhering to ISO 14971:2019, manufacturers not only fulfill necessary obligations under regulation medical devices but also position themselves for successful market access.
The risk management process outlined in ISO 14971:2019 consists of five key steps:
This comprehensive approach ensures that all potential risks are addressed effectively. Notably, the standard emphasizes that a risk management plan must include seven essential elements:
An optional eighth element can also be included to manage lifecycle aspects of the risk management plan.
Experts in the field stress the importance of viewing the risk management plan as a 'living document' that should be continuously updated as new information becomes available. Naveen Agarwal, Ph.D., emphasizes that "an organized approach is essential for good risk management," aligning with recent studies that highlight the necessity for improved risk management competencies within the Medtech sector. For example, qualitative interviews with risk management experts indicate that although Failure Mode Effect Analysis (FMEA) is the most commonly used tool, it frequently falls short in addressing safety risks during regular equipment usage, highlighting the necessity for a more effective risk management strategy.
Integrating effective risk management approaches throughout the healthcare equipment lifecycle is essential. Manufacturers are encouraged to adopt a proactive stance, ensuring that the regulation of medical devices includes risk management as a fundamental aspect of product development and commercialization rather than merely a compliance exercise. By doing so, they can improve their market preparedness and support the overall safety and effectiveness of healthcare products.
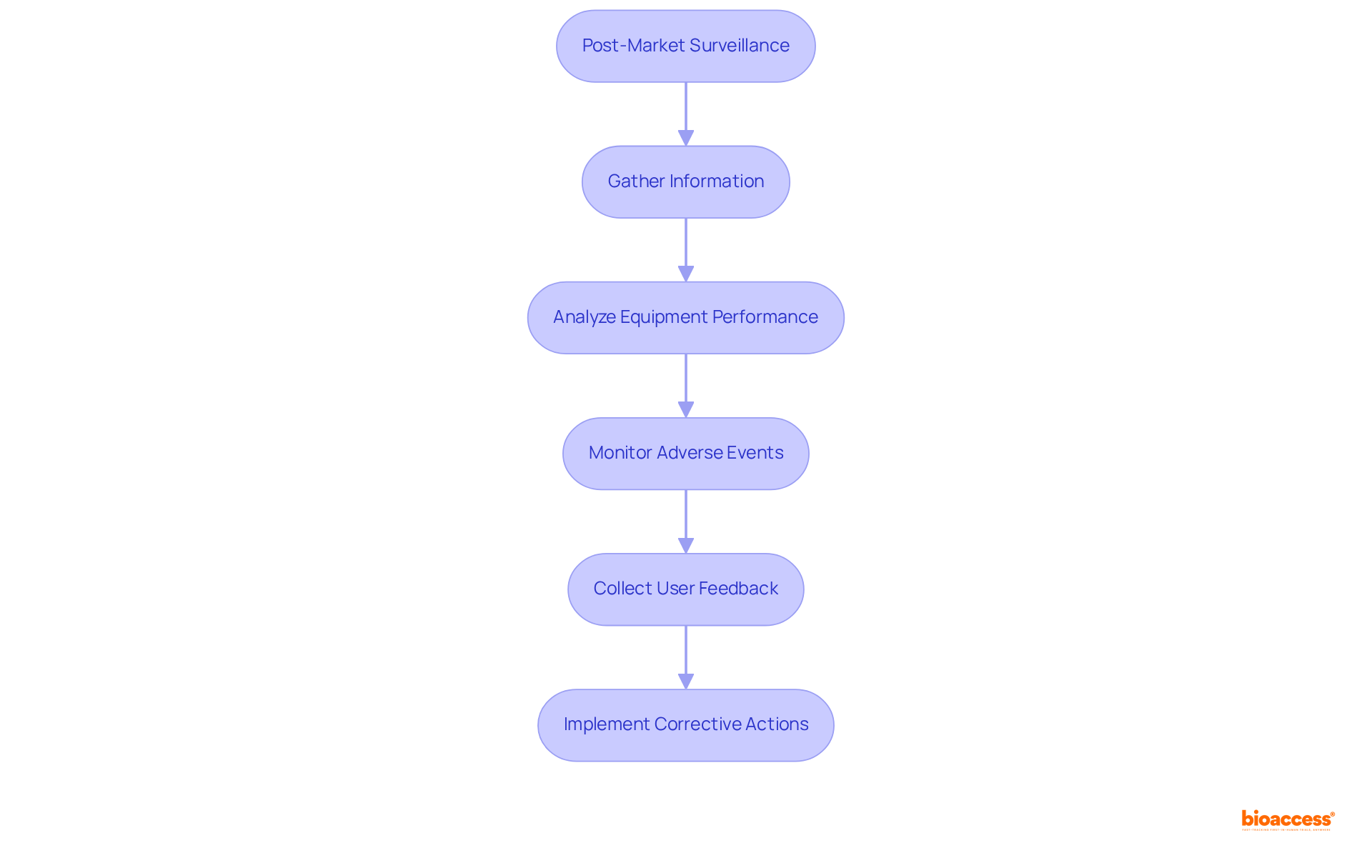
Post-market surveillance is essential in the medical product lifecycle, ensuring the continuous safety and effectiveness of products after they enter the market. Producers are mandated to systematically gather and analyze information regarding equipment performance, adverse events, and user feedback. This process is vital for identifying potential safety issues and implementing timely corrective actions, thereby upholding compliance with regulation medical devices and protecting patient safety.
Current trends indicate that nearly 33% of medical product adverse event reports submitted to the FDA experience delays, often exceeding six months after the producer is notified. This underscores the urgent need for improved reporting systems and proactive monitoring strategies. Effective post-market surveillance not only enhances product quality but also cultivates a culture of safety within organizations.
Industry experts highlight the necessity of robust monitoring strategies. For instance, William H. Maisel remarked, "The ideal measure of the success of the device approval or clearance processes would be the performance and reliability of each individual approved or cleared device." Additionally, the FDA's recent initiatives aim to enhance compliance with the regulation of medical devices by better integrating premarket and postmarket data, ensuring that producers remain vigilant in their monitoring efforts.
Data collection methods for post-market surveillance include utilizing the MAUDE database, which has been instrumental in tracking adverse events since 1996. The FDA receives approximately 200,000 case reports annually, illustrating the substantial volume of data being managed and the critical importance of effective surveillance. By leveraging advanced analytics and AI technologies, producers can optimize their reporting processes, ensuring prompt detection of safety issues and adherence to regulation medical devices.
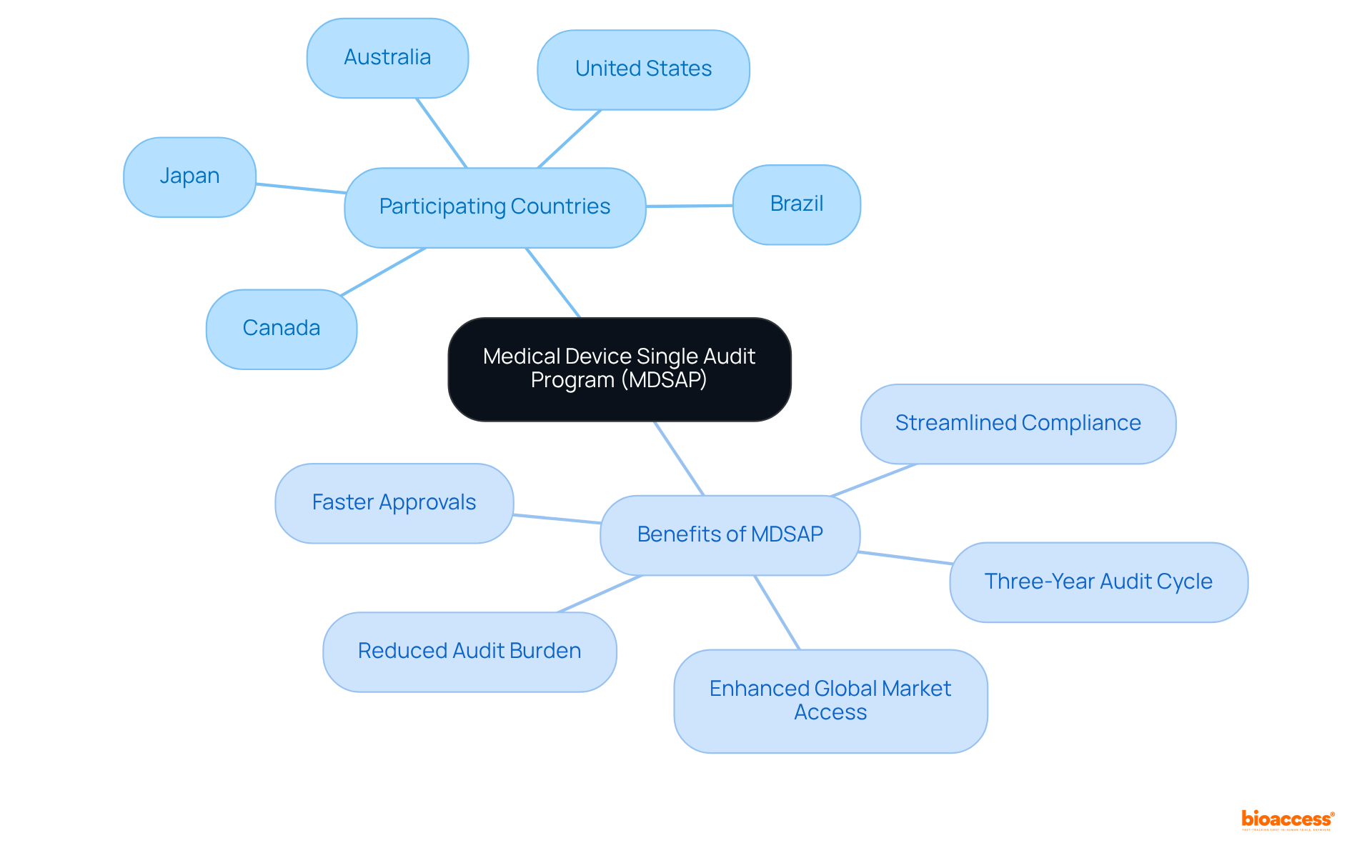
The Medical Device Single Audit Program (MDSAP) represents a pivotal advancement for producers in the healthcare equipment sector, allowing them to undergo a single regulatory audit that satisfies the requirements of multiple nations, including:
Since its introduction in 2014, MDSAP has significantly streamlined the compliance process, alleviating the burden of numerous audits on manufacturers. This program not only saves time and resources but also enhances global market access while ensuring strict adherence to regulation medical devices across various jurisdictions. By participating in MDSAP, companies can secure ethical approvals in as little as 4 to 6 weeks, achieving enrollment rates that are 50% faster than traditional markets, thereby positioning themselves for success in the competitive healthcare landscape. Furthermore, MDSAP operates on a three-year audit cycle, which further simplifies compliance and supports sustained regulatory adherence.
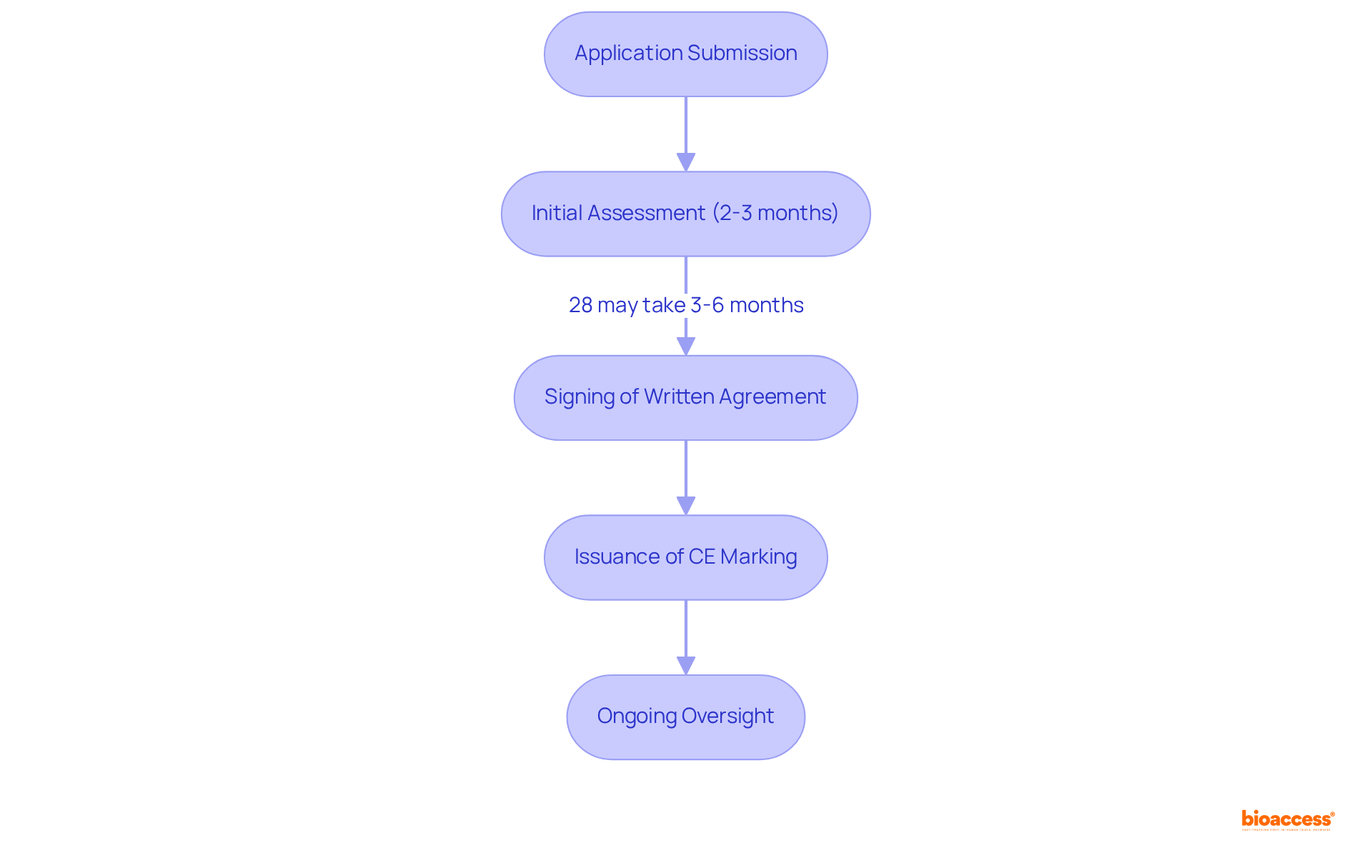
Notified bodies are organizations appointed by EU member states to evaluate the compliance of medical products prior to their marketing. They play an essential role in the certification process for regulation medical devices, conducting audits and assessments to ensure that the equipment meets all regulatory requirements. Manufacturers must engage with notified bodies to obtain CE marking, making their role essential for successful market access in Europe.
The assessment process typically takes an average of 2-3 months from the submission of an application to the signing of a written agreement. However, 28% of notified bodies indicate that this timeframe can extend to 3-6 months due to various factors, including the intricacy of the product and the resources available at the notified body. As of March 2023, a total of 11,418 applications had been processed, with a significant increase in the number of MDR certificates issued, rising from 1,190 to 2,951.
Notified bodies are also responsible for ongoing oversight of producers post-certification to ensure continued adherence to regulatory requirements. This encompasses overseeing the performance of equipment in the market and carrying out regular evaluations. The importance of selecting a reputable notified body cannot be overstated, as their ethical commitment and operational integrity directly impact the certification process. As GMED emphasizes, "An NB’s reputation is often a reflection of how it operates and its ethical commitment."
With the upcoming deadlines for MDR and IVDR applications, manufacturers are urged to submit complete applications promptly to avoid delays and potential rejections. Companies must lodge applications by May 26, 2024, to remain eligible for the extended transition period. Interacting effectively with notified bodies is essential for managing the intricacies of regulation medical devices and securing prompt market entry. Furthermore, it is essential to recognize that more than 80% of self-certified IVDD products will require notified body involvement under the regulation medical devices IVDR, emphasizing the increasing significance of these organizations in the present regulatory environment.
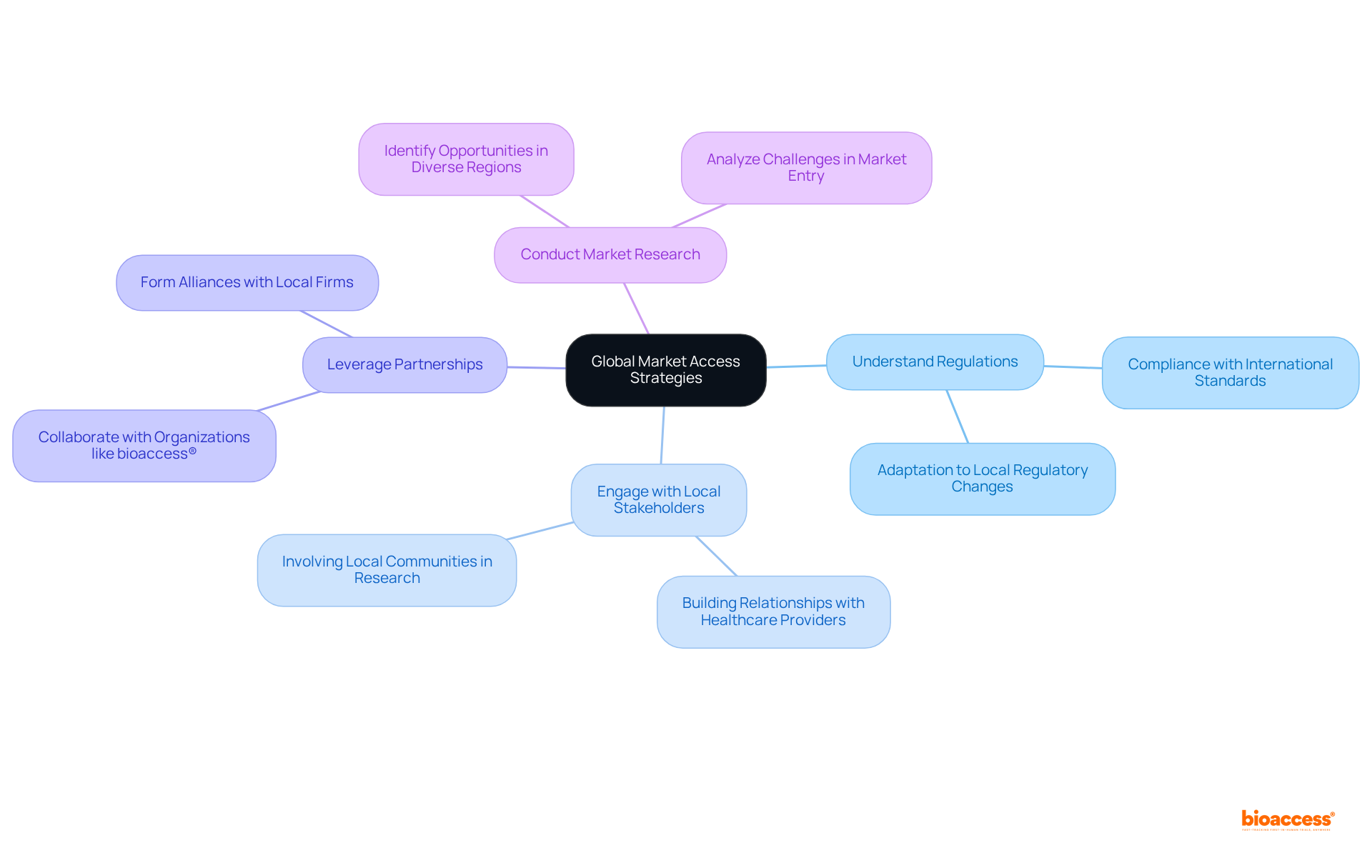
To enhance global market access, medical device producers must adopt a strategic approach that encompasses:
Conducting thorough market research to identify opportunities and challenges in diverse regions is essential. Furthermore, manufacturers should prioritize compliance with international standards and regulation of medical devices, as this facilitates smoother entry into new markets. This comprehensive strategy not only positions producers effectively but also underscores the importance of collaboration in navigating the complex Medtech landscape.
The journey to successful market access for medical devices is intricately woven with a multitude of regulations and standards that ensure safety, efficacy, and compliance. By understanding and navigating the complexities of regulations such as the Medical Device Regulation (MDR), In Vitro Diagnostic Regulation (IVDR), and ISO standards, manufacturers can position themselves effectively in a competitive landscape. The commitment to adhering to these regulatory frameworks not only facilitates market entry but also enhances product quality and patient safety.
Key insights from the article highlight the importance of strategic approaches, such as:
Furthermore, the emphasis on post-market surveillance underscores the necessity for ongoing vigilance in monitoring product performance and safety, reinforcing the notion that regulatory compliance is an ongoing commitment rather than a one-time hurdle.
As the landscape of medical device regulations continues to evolve, staying informed and adaptable is crucial for manufacturers aiming for success. Embracing these regulations as integral components of business strategy will not only pave the way for smoother market access but also foster trust and reliability in the healthcare sector. The call to action for industry stakeholders is clear:
What is bioaccess® and how does it assist Medtech and Biopharma firms?
bioaccess® leverages its extensive knowledge of medical device regulations to help Medtech and Biopharma firms achieve compliance with health product regulations, accelerating the approval process and enhancing the quality of clinical research.
How does bioaccess® expedite compliance in different regions?
bioaccess® utilizes the regulatory efficiency of Latin America, where ethical approvals can be secured in 4-6 weeks, and engages with diverse patient populations in the Balkans, alongside streamlined pathways in Australia, to navigate the complex compliance landscape.
What is the projected growth of regulatory affairs services by 2030?
The demand for regulatory affairs services is projected to reach USD 30.16 billion by 2030, expanding at a compound annual growth rate (CAGR) of 9.1%.
What are the key requirements of the Medical Device Regulation (MDR) for EU market access?
The MDR requires comprehensive technical documentation, robust risk management procedures, and effective post-market surveillance strategies to ensure product safety and performance.
Why is CE marking important for medical devices in the EU?
CE marking signifies compliance with EU safety standards and is essential for legal market entry, fostering trust and acceptance among consumers and healthcare providers.
What challenges do manufacturers face under the MDR?
Manufacturers face significant challenges due to stringent criteria for CE marking, with the validation period extending to 13-18 months and 81% of respondents finding the MDR complex.
What is the In Vitro Diagnostic Regulation (IVDR) and its significance?
The IVDR establishes compliance standards for diagnostic instruments, focusing on performance evaluation plans and requiring robust clinical evidence to demonstrate safety and effectiveness.
How has the IVDR impacted the involvement of notified bodies in the approval process?
Under the IVDR, the proportion of in vitro diagnostics requiring notified body involvement has increased from about 20% to 80-90%, necessitating closer collaboration between producers and notified bodies.
What are the classes of devices under the IVDR and their implications?
The IVDR categorizes devices into four classes (A, B, C, and D), with increasing levels of scrutiny corresponding to the associated risks, emphasizing the need for thorough risk analysis.
What role does clinical evidence play in IVDR compliance?
Clinical evidence is critical for demonstrating the safety and performance of diagnostic devices, and about 40% of initial protocol submissions require substantial revisions after regulatory or ethics committee review.
ISO14971:2019 Detailed Analysis and Periodic Safety Update Report Establishment Method for the Single Use Medical Device - Focusing on Medical Device Regulation 2017/745 requirements -Journal of Biomedical Engineering Research
| Korea Science (https://koreascience.kr/article/JAKO202309560969049.page)
Statistical Procedures for the Medical Device Industry - Taylor Enterprises (https://variation.com/product/statistical-procedures-for-the-medical-device-industry)
Failure mode effect analysis use and limitations in medical device risk management (https://sciencedirect.com/science/article/pii/S2199853124002336)
ISO 14971 Fundamentals: Risk management plan (https://naveenagarwalphd.substack.com/p/iso-14971-fundamentals-risk-management-plan)