


The article presents effective strategies to enhance clinical trial diversity, underscoring the critical need for inclusive recruitment and community engagement. It delineates nine key strategies, including:
These strategies are designed to foster trust and increase participation among underrepresented populations, ultimately improving the overall quality and relevance of clinical research outcomes.
Enhancing diversity within clinical trials transcends regulatory requirements; it is a pivotal element in ensuring that medical research accurately reflects the diverse populations it seeks to serve. By adopting strategies that foster inclusive recruitment, community engagement, and cultural competency, researchers can unlock the potential for more comprehensive and effective health outcomes. Yet, the challenge persists: how can the industry effectively implement these strategies to dismantle historical barriers and cultivate trust among underrepresented groups? This article delves into nine actionable strategies that can significantly enhance clinical trial diversity, paving the way for a more equitable healthcare landscape.
bioaccess® expertly navigates the regulatory landscape of Latin America, engages with the diverse patient populations of the Balkans, and utilizes Australia's efficient pathways to expedite ethical approvals and enrollment processes. By strategically conducting clinical studies in these regions, bioaccess® significantly enhances clinical trial diversity, ensuring that research encompasses a broad demographic range. This approach not only accelerates the testing schedule—enrollment is 50% faster than in conventional markets—but also enriches the data collected, resulting in more comprehensive and relevant outcomes across varied populations.
Notable initiatives, such as the CardioMonitor study in Chile, which achieved an impressive 98% accuracy in arrhythmia detection, illustrate the advantages of diverse participant pools. Industry leaders emphasize that clinical trial diversity in inclusive research leads to improved health outcomes, underscoring the critical importance of diversity in medical studies.
By fostering collaboration with local communities and employing culturally appropriate methodologies, including community-centered research to cultivate trust and encourage participation among local populations, bioaccess® is committed to enhancing clinical trial diversity in the field of medical research. This dedication ultimately contributes to more equitable healthcare solutions, addressing the historical mistrust that marginalized groups have experienced in the realm of medical research.
To broaden participant demographics and improve clinical trial diversity, clinical study sponsors must adopt inclusive recruitment strategies that proactively engage underrepresented groups. This approach necessitates focused engagement in frequently neglected areas, the use of culturally relevant messaging, and the employment of diverse recruitment teams that reflect the populations being studied to improve clinical trial diversity.
As Gerald S. Bloomfield emphasizes, "engagement initiatives enhance awareness and involvement in studies among minority groups, with certain programs boosting enrollment by more than 60%." Simplifying eligibility standards can further attract a broader range of participants, ensuring that studies more accurately reflect the general population.
By cultivating trust and developing connections within these groups, as Bloomfield notes, "earning trust requires time and effort," sponsors can significantly boost participation rates and ultimately enhance the generalizability of study outcomes. Furthermore, the FDA's initiative for at least 20% underrepresented group participation by 2025 underscores the urgency of implementing inclusive strategies to promote clinical trial diversity.
Addressing obstacles to involvement, such as cultural mistrust and logistical challenges, is essential for achieving fair representation in research studies.
Involving populations through outreach initiatives, informational meetings, and collaborations with local entities is essential for building trust in clinical studies. By actively engaging local leaders and stakeholders in the planning and implementation of studies, researchers can effectively tackle concerns, dispel misconceptions, and foster a sense of ownership among prospective participants. This joint approach not only enhances participation rates but also guarantees that experiments are customized to address the particular requirements of the group.
For instance, studies have shown that sites receiving cultural safety training from COUCH Health enrolled 26% more diverse patients compared to those that did not. Furthermore, outreach efforts that included collaboration with Federally Qualified Healthcare Centers (FQHCs), where COUCH Health referred patients to the study, successfully screened a diverse patient pool, with 78% of screened patients coming from varied backgrounds. Such initiatives demonstrate that when groups are involved, the chances of participation rise considerably, ultimately resulting in more representative research outcomes and greater clinical trial diversity.
Significantly, just 32% of patients indicated that their physicians had communicated details about clinical studies with them, emphasizing the essential requirement for improved dialogue via community involvement.
Offering cultural competency training for research teams is essential for enhancing engagement with diverse populations and promoting clinical trial diversity. This training should encompass critical topics such as:
By equipping researchers with these vital skills, studies can foster clinical trial diversity, creating a more welcoming environment for participants and ultimately leading to increased enrollment and retention.
Forming alliances with local entities—such as health centers, advocacy organizations, and cultural institutions—can significantly enhance recruitment efforts for research studies. These organizations often have established trust within their communities, enabling researchers to connect with potential contributors more effectively. By collaborating on outreach initiatives and educational programs, clinical trial sponsors can elevate awareness and understanding of the trials, consequently leading to higher participation rates.
Notably, 70% of potential participants reside more than two hours away from study centers, complicating participation and underscoring the necessity for local partnerships to address logistical barriers. Local leaders emphasize that such collaborations not only facilitate access to diverse populations but also foster a sense of ownership and involvement among residents, which is essential for successful recruitment.
As Luther T. Clark, M.D. states, "Building trust between researchers and trusted community stakeholders in minority communities is critical for overcoming barriers." By leveraging the insights and connections of these local organizations, research sponsors can cultivate a more inclusive investigative environment that promotes clinical trial diversity and reflects the diversity of the communities they aim to support.
Moreover, with fewer than 20% of clinical studies in the U.S. achieving their recruitment goals, effective collaborations are paramount for enhancing recruitment success.
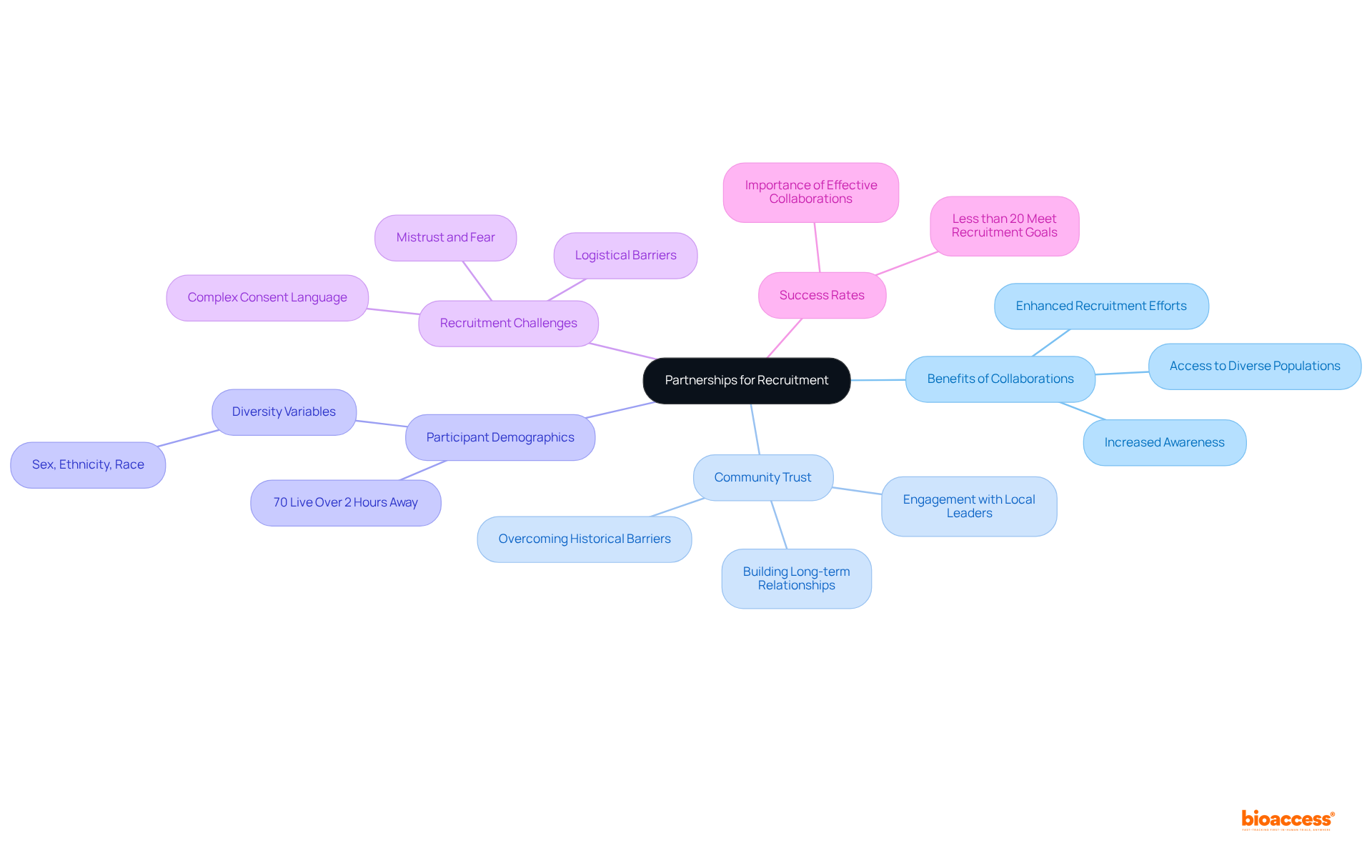
Clear communication regarding study procedures, including informed consent, potential risks, and the advantages of participation, is essential for fostering participant trust. Researchers must provide clear and accessible information in diverse formats and languages to guarantee comprehensive understanding. Regular updates on the progress and results of the study not only bolster trust but also encourage ongoing involvement, which is crucial for enhancing clinical trial diversity, particularly among diverse groups.
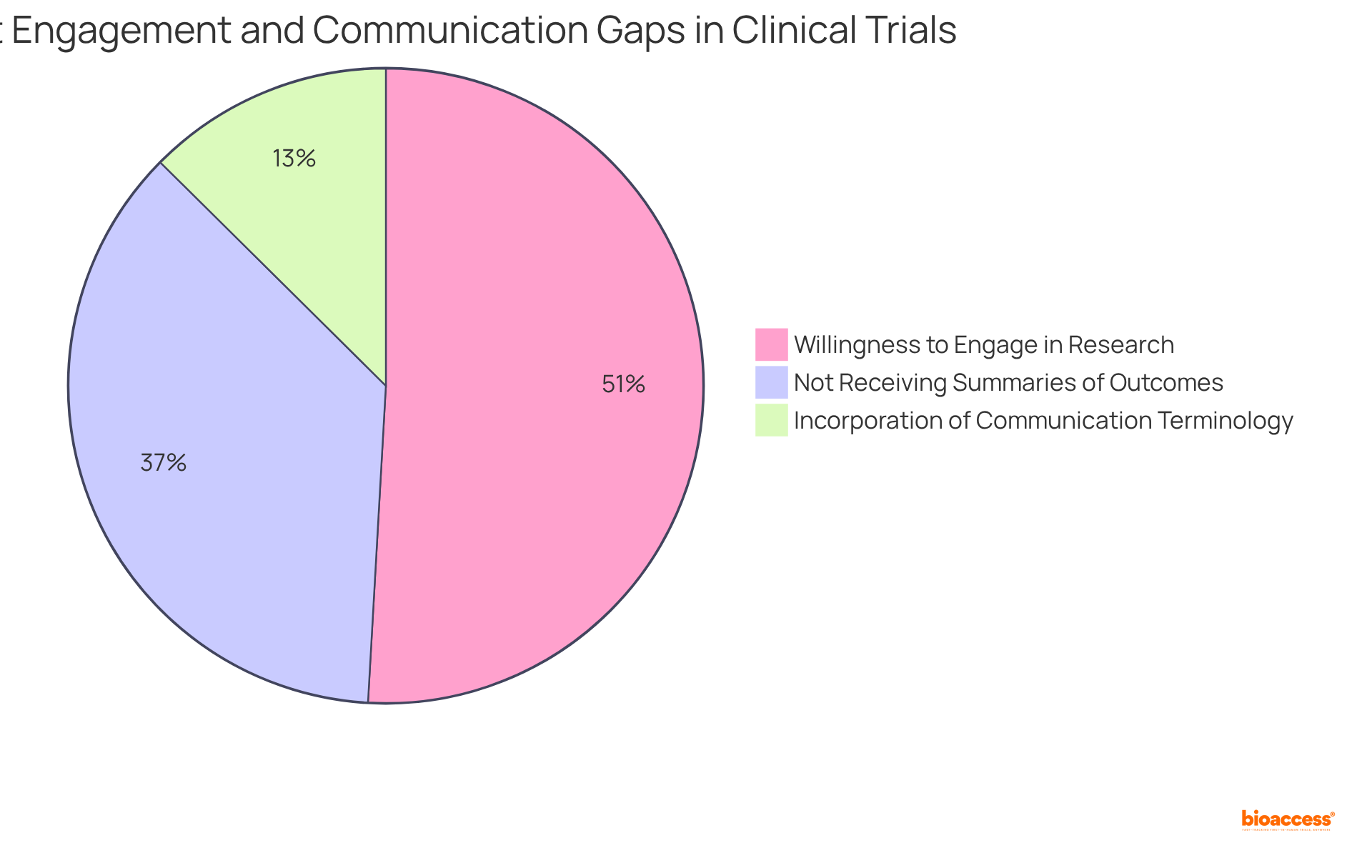
As highlighted by research specialists, effective communication strategies significantly impact recruitment and retention rates, ultimately enhancing the overall success of studies. For instance, a survey indicated that:
Furthermore, only 21% of reviewed research protocols incorporated communication-related terminology, revealing existing gaps in communication practices. By prioritizing transparency and clarity, researchers can cultivate a more inclusive environment that empowers contributors and promotes health equity, particularly in light of recent funding cuts that threaten studies focused on underrepresented groups and the importance of clinical trial diversity.
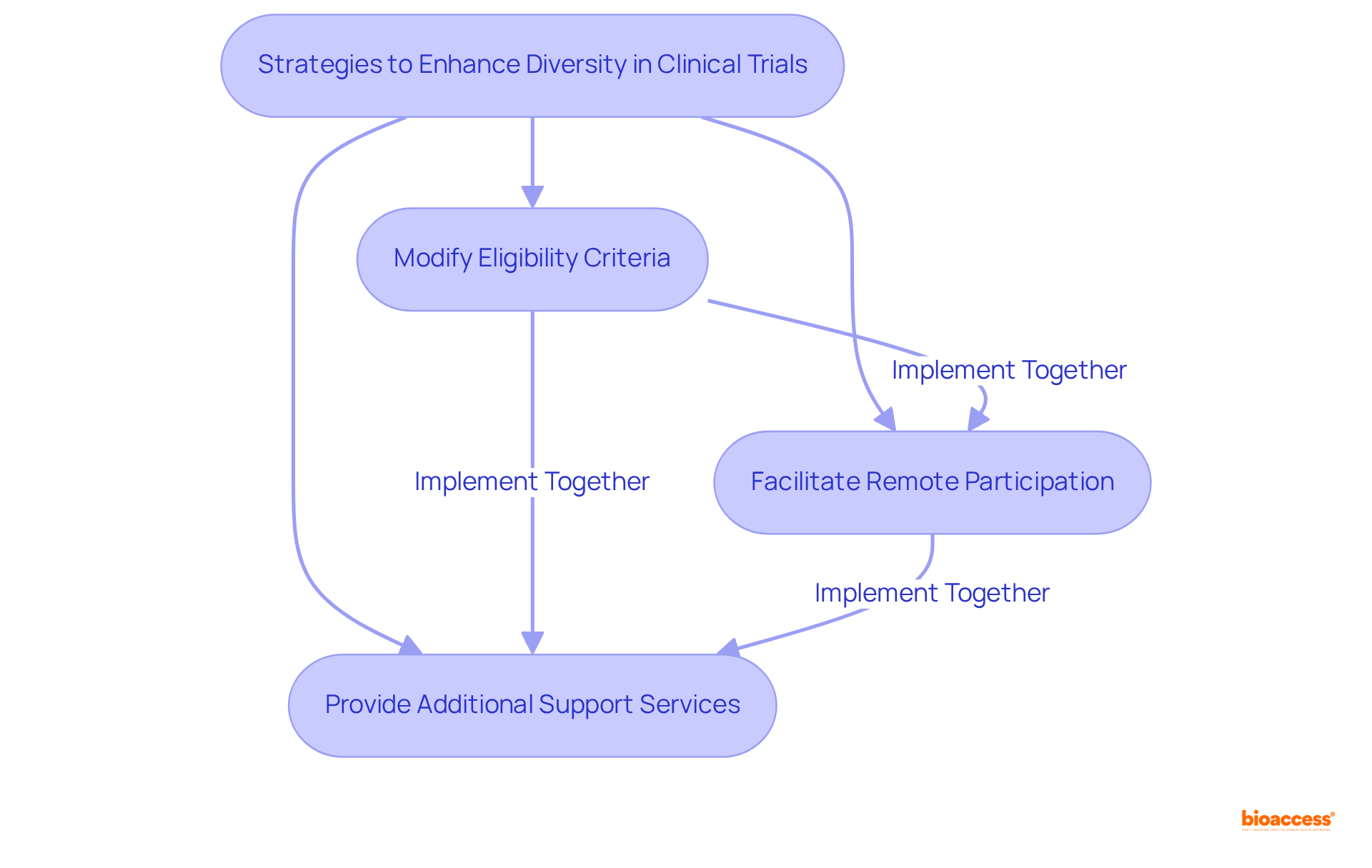
To promote clinical trial diversity in research studies, it is crucial to establish flexible study protocols that meet the diverse requirements of the individuals involved. This may involve:
Recent data indicates that Walgreens has engaged over 4 million patients for potential enrollment in research studies, with more than 60% being women, highlighting the significance of varied participation. By addressing the unique challenges faced by different demographic groups, researchers can foster clinical trial diversity, thereby creating a more inclusive environment that encourages engagement from a wider array of individuals.
As Linda Goler Blount, president and CEO of the Black Women’s Health Imperative, asserts, "When we say something is evidence-based, I want the women I’m talking to to know that the standard of care was created with them in mind and that they were involved in the research." Such strategies not only enhance clinical trial diversity but also enrich the quality of research outcomes by ensuring that findings are reflective of the broader population.
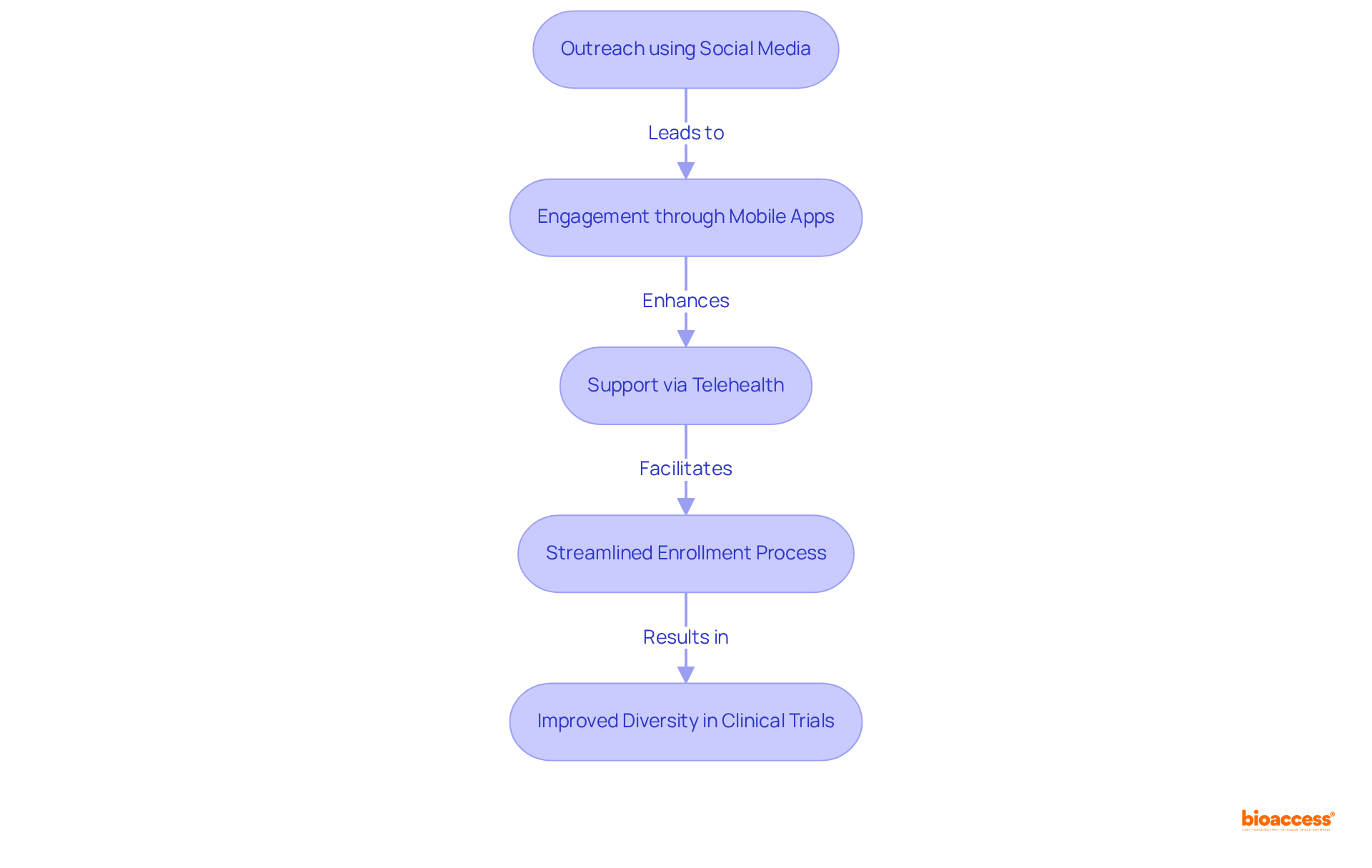
Utilizing technology—such as social media, mobile applications, and telehealth platforms—can significantly enhance clinical trial diversity by improving the involvement and recruitment of varied research participants. Digital tools not only improve outreach to underrepresented groups but also contribute to clinical trial diversity by streamlining the enrollment process and providing ongoing support throughout the study.
For instance, 73% of patients prefer to learn about clinical research opportunities from their doctor's office, while 42% express interest in hearing from advocacy groups. By leveraging these technologies, researchers can broaden their reach and foster a more participant-friendly experience.
Social media, in particular, has shown considerable potential in engaging diverse individuals, with younger demographics more likely to discover trials through these platforms. Successful campaigns have employed targeted messaging that resonates with specific communities, thereby enhancing participation rates.
Furthermore, mobile applications can deliver real-time updates and resources, ensuring participants feel supported and informed throughout their journey. As digital tools continue to evolve, they will play a crucial role in promoting clinical trial diversity in research studies, ultimately leading to more representative and effective outcomes.
Notably, 85% of medical studies fail to recruit sufficient participants, and 80% are postponed or terminated due to recruitment challenges, underscoring the necessity for improved strategies. As Brant emphasizes, while digital tools enhance recruitment efficiency, maintaining a human touch is essential for engaging and retaining individuals.
To ensure ongoing commitment to clinical trial diversity in research trials, it is essential to regularly monitor and evaluate diversity metrics. This process involves tracking participant demographics, recruitment strategies, and retention rates. By analyzing this data, researchers can pinpoint areas for improvement and adapt their approaches accordingly. Such ongoing assessment not only demonstrates responsibility but also fosters a culture of inclusivity within healthcare research. It is imperative that researchers take these steps to promote clinical trial diversity and enhance the integrity of clinical trials.
Offering ongoing education on diversity matters for all research stakeholders—including investigators, sponsors, and community allies—is crucial for promoting a culture of inclusivity. Training programs should cover topics such as implicit bias, cultural competency, and the importance of diverse representation in research.
For instance, only 3% of full-time educators at educational institutions are Hispanic, and under 5% are African American, emphasizing the urgent necessity for diversity in research studies. By equipping stakeholders with the knowledge and resources to foster clinical trial diversity, clinical studies can become more inclusive and effective in addressing the needs of varied populations.
As Rachael Evans notes, inclusive language helps individuals understand and respect each other's unique experiences, affirming their sense of belonging. Furthermore, fostering an understanding of cultural differences can lead to more respectful and effective communication with participants, ultimately enhancing the overall trial experience.
As highlighted by diversity trainers, creating an environment where all voices are valued not only enriches the research process but also drives better health outcomes for all communities involved.
Enhancing clinical trial diversity stands as a pivotal step toward achieving equitable healthcare outcomes, transcending mere ethical considerations. By implementing strategic approaches—such as inclusive recruitment, community engagement, cultural competency training, and leveraging technology—the clinical research landscape can be transformed to accurately mirror the diverse populations it seeks to serve. This unwavering commitment to diversity is essential for generating data that is both comprehensive and relevant across various demographic groups.
Key strategies underscore the importance of:
Furthermore, continuous education for all stakeholders involved in clinical trials is crucial to ensure that diversity remains a priority, fostering an inclusive environment that encourages participation from underrepresented groups. The successful execution of these strategies can significantly enhance enrollment rates and improve the overall quality of research outcomes.
Ultimately, the call to action is unequivocal: embracing diversity in clinical trials is vital for advancing medical research and ensuring that all communities reap the benefits of healthcare innovations. By prioritizing these strategies, researchers and sponsors can significantly contribute to a more equitable future in healthcare, where every voice is heard, and every individual has the opportunity to shape the medical advancements of tomorrow.
What is bioaccess® and how does it enhance clinical trial diversity?
bioaccess® is an organization that navigates the regulatory landscape in Latin America, engages diverse patient populations in the Balkans, and utilizes efficient pathways in Australia to expedite ethical approvals and enrollment processes. By strategically conducting clinical studies in these regions, bioaccess® enhances clinical trial diversity, leading to faster enrollment and more comprehensive data across varied populations.
How much faster is the enrollment process in clinical trials conducted by bioaccess® compared to conventional markets?
Enrollment in clinical trials conducted by bioaccess® is 50% faster than in conventional markets.
Can you provide an example of a successful initiative by bioaccess®?
The CardioMonitor study in Chile is a notable initiative, achieving 98% accuracy in arrhythmia detection, which illustrates the advantages of having a diverse participant pool.
What strategies can clinical study sponsors use to improve participant demographics?
Clinical study sponsors can adopt inclusive recruitment strategies that engage underrepresented groups, utilize culturally relevant messaging, and employ diverse recruitment teams. Simplifying eligibility standards can also help attract a broader range of participants.
Why is it important to engage underrepresented groups in clinical trials?
Engaging underrepresented groups enhances awareness and involvement in studies, leading to improved participation rates and more generalizable study outcomes. The FDA has set a goal for at least 20% participation from underrepresented groups by 2025, highlighting the urgency of this issue.
What role does community engagement play in clinical trial participation?
Community engagement through outreach initiatives, informational meetings, and collaborations with local leaders fosters trust and participation. Actively involving local stakeholders helps address concerns and ensures studies are tailored to the specific needs of the community.
How effective are cultural safety training and outreach efforts in increasing diversity in clinical trials?
Cultural safety training, such as that provided by COUCH Health, has been shown to increase the enrollment of diverse patients by 26%. Outreach efforts that collaborate with Federally Qualified Healthcare Centers (FQHCs) have successfully screened a diverse patient pool, with 78% of screened patients coming from varied backgrounds.
What percentage of patients reported that their physicians discussed clinical studies with them?
Only 32% of patients indicated that their physicians had communicated details about clinical studies, highlighting the need for improved dialogue through community involvement.