


The landscape of clinical trials is undergoing a significant transformation through the adoption of Electronic Data Capture (EDC) systems. These innovative digital solutions streamline data management, enhance accuracy, and ensure compliance with rigorous regulatory standards. As the healthcare industry faces increasing demands for efficient data handling, EDC systems emerge as essential tools that offer real-time insights and facilitate seamless integration with other digital health technologies.
By addressing common challenges associated with traditional paper-based methods, EDC systems not only improve the quality of clinical data but also contribute to participant safety and the overall integrity of research outcomes. This article delves into the myriad benefits, key features, and future trends of EDC systems, highlighting their critical role in shaping the future of clinical research.
'Electronic Information Gathering (EIG) platforms transform the environment of research trials by greatly improving information collection procedures, reducing mistakes, and increasing overall operational effectiveness.'. Moving from conventional paper methods to advanced digital platforms enables researchers to attain enhanced information precision and dependability. This change not only simplifies information management but also tackles common challenges related to current clinical information solutions, such as quality problems and cumbersome study setups.
'EDC systems offer immediate access to information, which is essential for prompt decision-making and efficient oversight of trial progress.'. 'As stated by the World Health Organization, with more than two million medical devices utilized worldwide, the significance of high-quality information management is crucial as it protects participant safety and guarantees that the conclusions derived from the analysis are valid and dependable.'. 'Article 62 of the European Union Medical Device Regulation emphasizes that clinical investigations must prioritize the rights and well-being of participants while ensuring that the generated clinical information is scientifically valid and robust.'.
Furthermore, the benefits of EDC frameworks go beyond simple information gathering. They facilitate the integration of information from various sources, including electronic health records and real-world information, which can yield valuable insights into participants' health and contextual factors that influence trial outcomes. The current regulatory landscape, particularly concerning Software as a Medical Device (SaMD), is evolving rapidly, with the market projected to grow at a compound annual growth rate of approximately 52% from 2023 to 2028. This growth highlights the importance of strong information frameworks that can adjust to regulatory requirements while offering thorough management solutions.
Ultimately, embracing electronic collection methods not only lessens the challenges linked to manual information management but also greatly cuts down research and development expenses. 'This transition is essential for sponsors seeking to improve adherence to strict regulations while ensuring the precision and dependability of research information, ultimately benefiting both the study's integrity and participant safety.'.
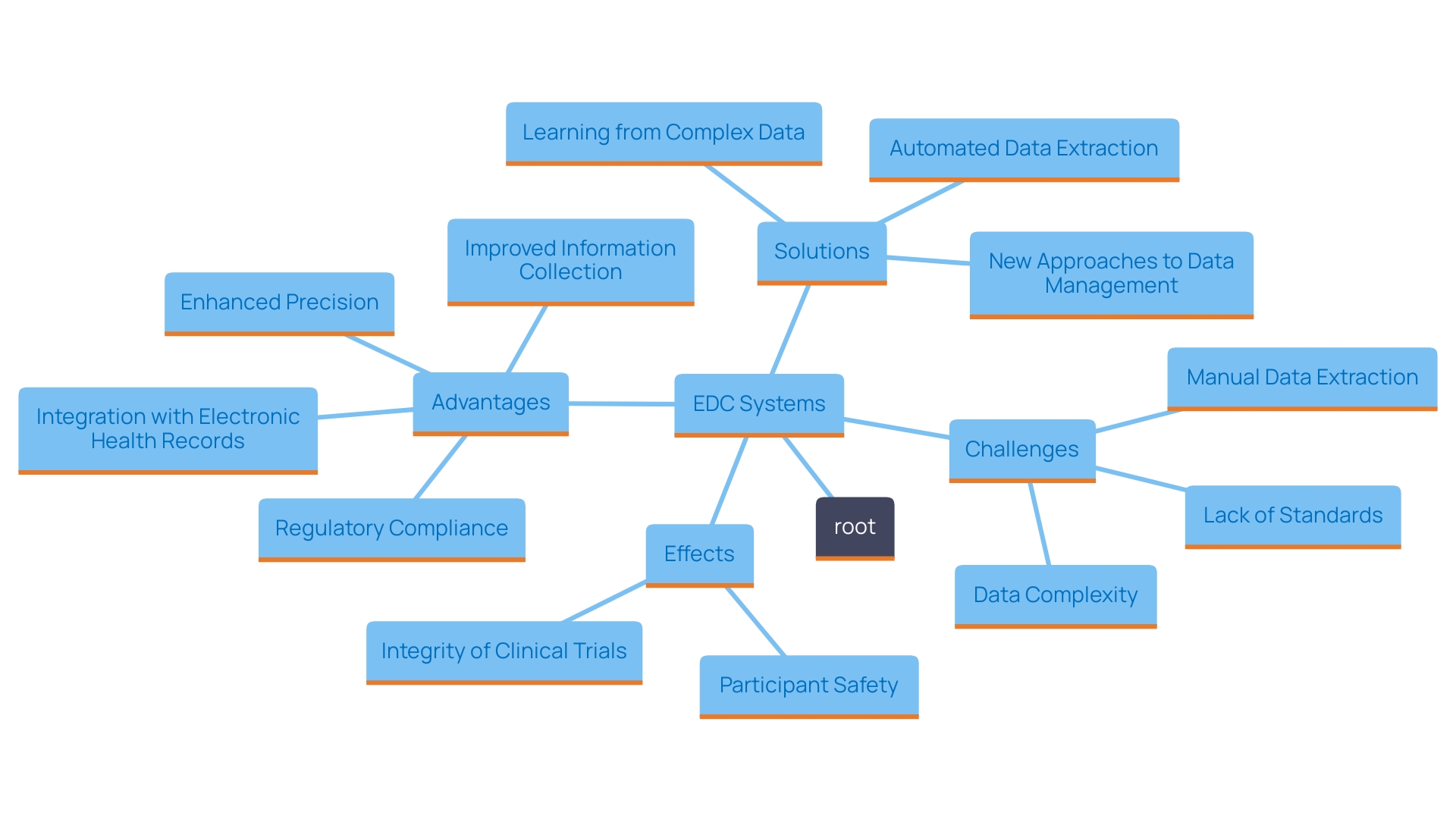
'Electronic Data Capture (EDC) platforms play a vital role in the contemporary research environment by simplifying data gathering and administration processes.'. 'These frameworks are characterized by user-friendly interfaces, enabling researchers to navigate complex workflows with ease.'. 'Customizable workflows provide flexibility designed for particular study needs, ensuring that it can adjust to different clinical trial protocols.'.
An essential characteristic of EDC platforms is their assistance for electronic case report forms (eCRFs), which enable immediate information entry and monitoring. This capability is complemented by automatic information validation, which enhances integrity by identifying discrepancies or errors at the point of entry. Moreover, EDC solutions are designed to integrate seamlessly with other digital health technologies, such as intelligent document processing (IDP). This integration enables the handling of unstructured and semi-structured information from various healthcare documents, enhancing overall management efficiency.
The significance of these sophisticated frameworks is highlighted by the necessity for adherence to standards such as ISO 14155:2020, which stresses the importance of strong management of medical information. According to estimates from the World Health Organization (WHO), there are more than two million various medical devices available, highlighting the need for thorough information collection and examination to guarantee the safety and efficacy of these products. 'EDC solutions not only streamline the information gathering process but also offer critical reporting features that assist in informed decision-making in research studies, ultimately improving patient outcomes and furthering medical knowledge.'.
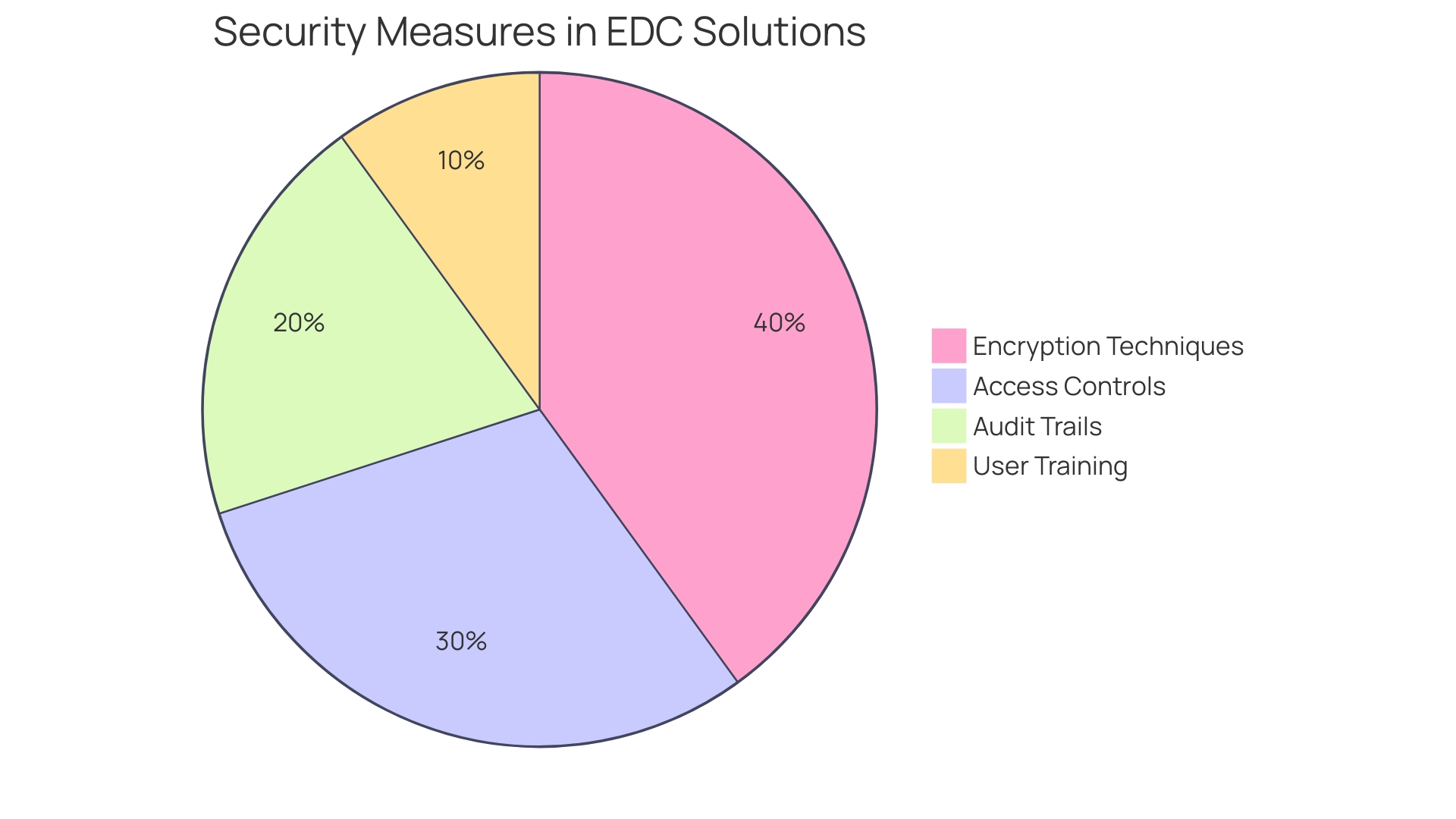
Ensuring information security and regulatory compliance is crucial in the realm of clinical trials, where the stakes involve both participant safety and the integrity of medical advancements. Electronic Data Capture (EDC) solutions are designed with multiple layers of security, employing advanced encryption techniques, strict access controls, and comprehensive audit trails to safeguard sensitive patient information. These systems are not just tools; they represent the dedication to upholding adherence to strict regulations like HIPAA and GDPR, thus offering stakeholders assurance in the integrity and confidentiality of the information gathered.
'The significance of strong health information management is highlighted by the World Health Organization's estimate that there are over two million different kinds of medical devices in circulation globally, each potentially affecting numerous lives.'. As emphasized in Article 62 of the European Union Medical Device Regulation (EU MDR), the design and execution of clinical investigations must prioritize the rights, safety, and well-being of participants, ensuring that the clinical information generated is both valid and reliable.
Moreover, the financial implications of insufficient information protection are significant. Research from IBM indicates that the global average expense of a breach is approximately $4.45 million, reflecting a 15% increase over the last three years. Beyond the immediate financial impact, breaches can tarnish brand reputation, diminish consumer trust, and jeopardize the safety of research subjects.
In a competitive environment where the life sciences, biotechnology, and pharmaceutical sectors are rapidly evolving, organizations that prioritize information security position themselves advantageously. A secure and ethical approach to information management is not just a regulatory requirement; it is integral to fostering collaboration among stakeholders. By safeguarding confidential information throughout the research process, organizations not only adhere to legal standards but also enhance the overall welfare and privacy of individuals participating in medical studies.
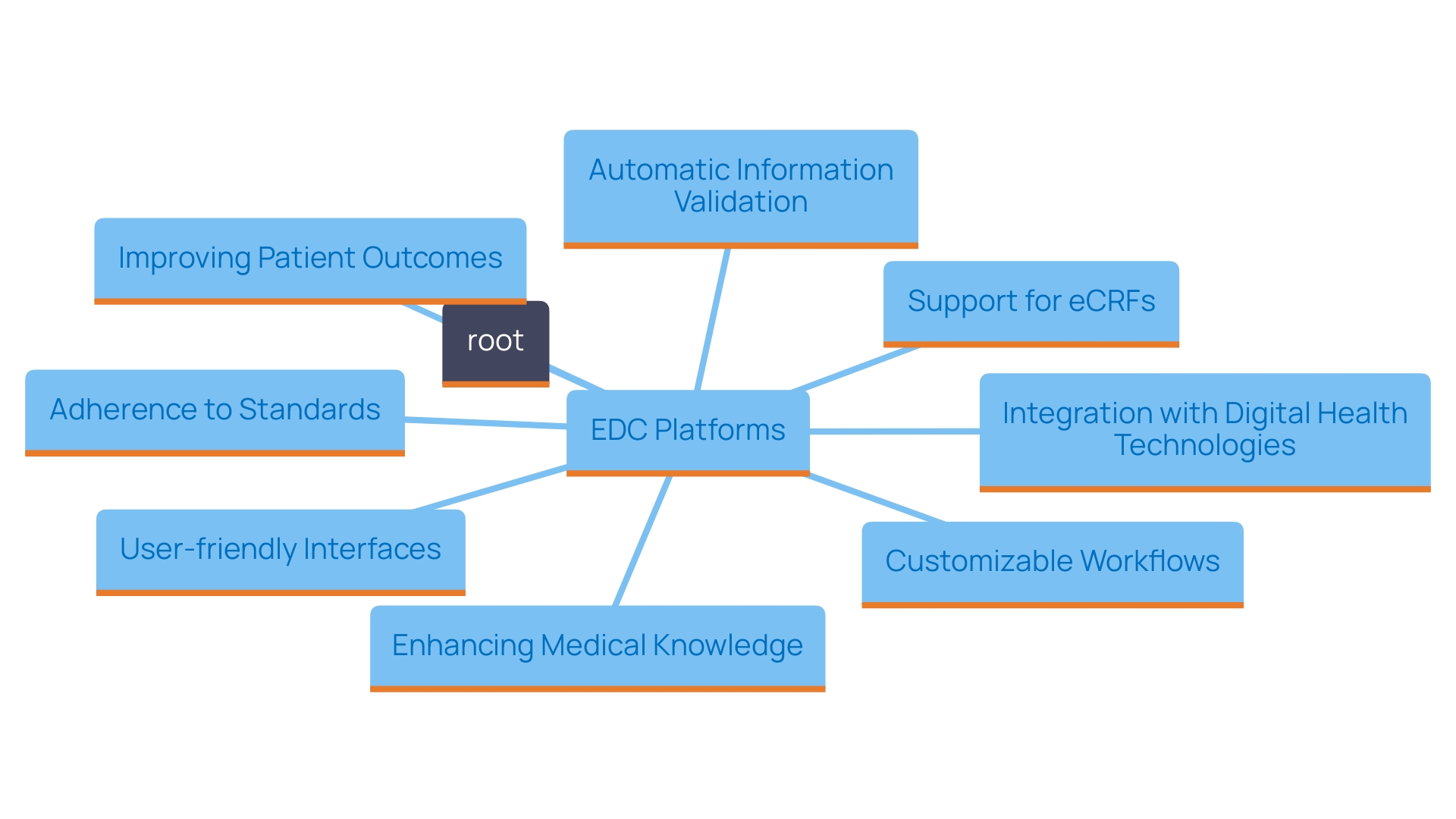
The incorporation of Electronic Information Collection (EDC) tools in research studies offers a revolutionary method for handling information, greatly improving both effectiveness and precision. By automating the information collection process, EDC systems reduce the dependence on manual entry, a frequent source of mistakes that can jeopardize trial integrity. This change not only lessens the chance of errors but also speeds up information processing schedules, enabling research studies to advance more rapidly.
As the World Health Organization (WHO) indicates, there are approximately two million different medical devices available worldwide, emphasizing the critical need for careful information management in research involving human subjects. The safety and effectiveness of these devices, which may ultimately affect millions of patients, depend on the quality of gathered information from trials. Rules like Article 62 of the European Union Medical Device Regulation (EU MDR) require that research trials prioritize the rights and safety of participants while ensuring that the produced information is scientifically valid and trustworthy.
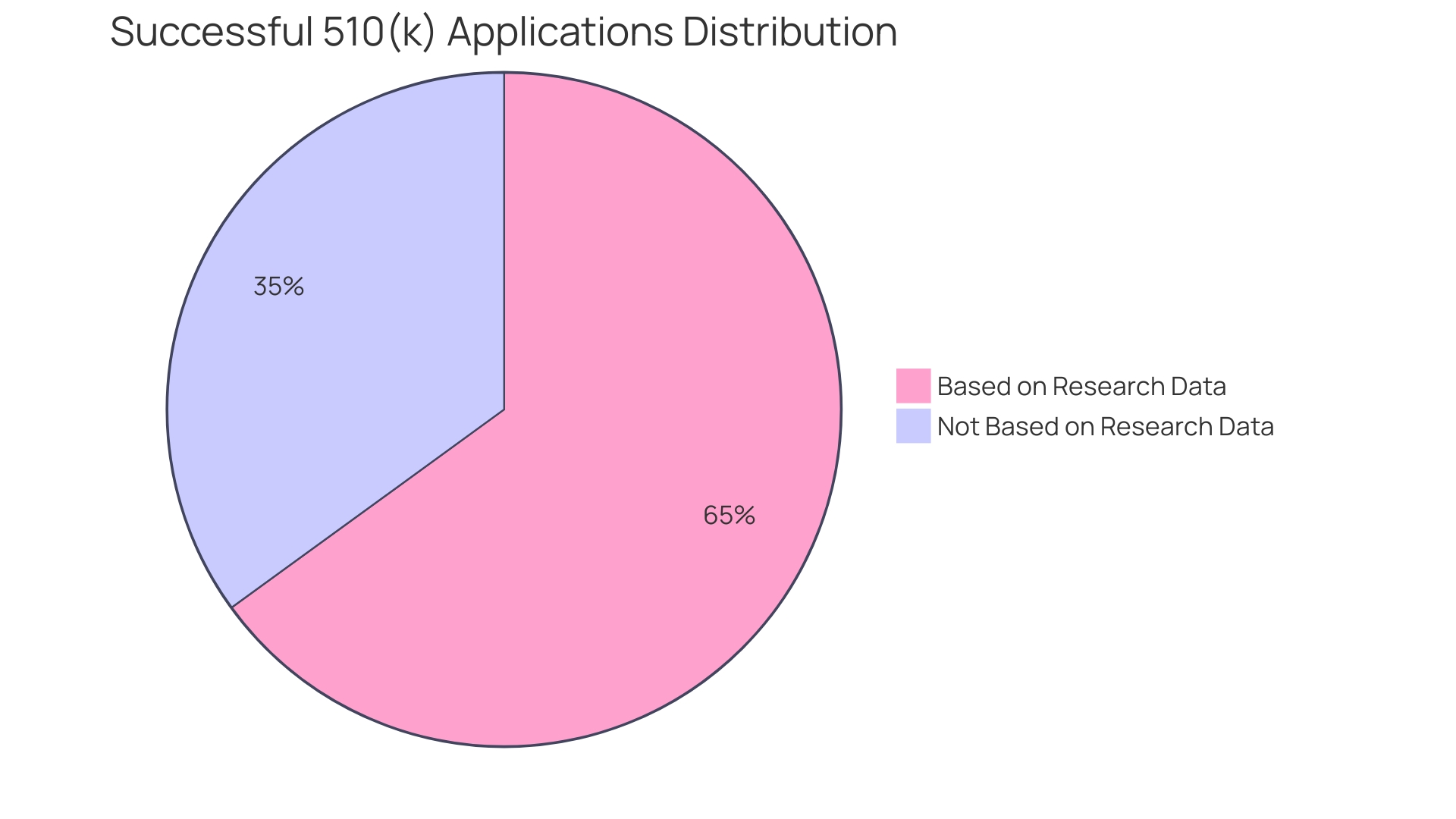
Furthermore, the efficiency achieved from EDC solutions translates directly into more effective resource allocation and streamlined study timelines. For example, in the United States, approximately 10-15% of successful 510(k) applications for Class II devices are founded on information gathered from research studies. In contrast, all Class III devices necessitate extensive trials to validate their safety and effectiveness. Hence, adopting EDC systems is not merely a technological upgrade; it is an essential strategy for meeting regulatory requirements and enhancing the overall robustness of clinical research operations.
The expanding market for Software as a Medical Device (SaMD) further emphasizes the significance of effective information management. With the Same sector projected to experience a compound annual growth rate of approximately 52% from 2023 to 2028, the demand for reliable data collection and analysis tools is more critical than ever. Regulatory professionals are increasingly called upon to navigate the complexities of diverse global regulations, making it essential that organizations utilize EDC platforms to ensure compliance and facilitate successful market entry for new digital health solutions.
With the rapid advancement of technology, Electronic Data Capture (EDC) platforms are poised to integrate sophisticated features such as artificial intelligence (AI) and machine learning (ML). These innovations promise to transform information analysis through predictive capabilities, allowing researchers to glean deeper insights from trial information. Importantly, AI can improve the effectiveness of information processing, greatly reducing the time and effort typically needed in information gathering, which is frequently carried out in separate frameworks that do not utilize available electronic health records or real-world information.
The integration of wearable gadgets and mobile applications into EDC frameworks will further enable real-time information collection, improving patient involvement throughout clinical trials. This integration allows for the gathering of extensive patient-reported information and details from daily living activities, which are frequently disregarded in conventional trials. By capturing a broader spectrum of health information, EDC systems can provide a more nuanced understanding of participants' health contexts, including social determinants that impact engagement and retention.
Moreover, the push for health data interoperability, supported by public investment and federal legislative measures like the Health Information Technology for Economic and Clinical Health Act, aims to standardize health data elements across platforms. This infrastructure is essential for developing reusable research trial capabilities, ultimately improving the validity and generalizability of trial outcomes. As the healthcare landscape evolves, the integration of these digital health technologies will be vital for improving the efficiency and effectiveness of clinical investigations.
The adoption of Electronic Data Capture (EDC) systems represents a pivotal advancement in clinical trials, enhancing data collection and ensuring regulatory compliance. By improving data accuracy and streamlining operations, these systems facilitate real-time access to crucial information, which is vital for participant safety and timely decision-making.
Key features such as customizable workflows and electronic case report forms contribute to efficient data management. The integration of EDC systems with other digital health technologies allows for comprehensive data handling, while adherence to regulations like ISO 14155:2020 emphasizes the importance of robust data practices in safeguarding participant welfare.
Data security is paramount, with EDC systems employing advanced measures to protect sensitive information. The financial implications of data breaches further underscore the need for secure data management, fostering trust within the life sciences sector.
Looking to the future, the integration of artificial intelligence and machine learning in EDC systems promises to enhance data analytics capabilities significantly. Additionally, the incorporation of wearable devices and mobile applications will facilitate real-time data capture, enriching the quality of clinical trials. As the healthcare landscape continues to evolve, EDC systems will play a crucial role in improving the efficiency and effectiveness of clinical investigations while ensuring compliance with complex regulatory requirements.
What are Electronic Data Capture (EDC) platforms?
EDC platforms are digital systems designed to streamline data collection and management in clinical research trials. They enhance information accuracy, reduce errors, and improve overall operational efficiency compared to traditional paper methods.
How do EDC platforms improve information management?
By transitioning to digital platforms, researchers can achieve greater precision and reliability in data collection. EDC systems simplify information management and address common issues with existing clinical data solutions, such as quality concerns and complex study setups.
What role do EDC systems play in decision-making during trials?
EDC systems provide immediate access to information, which is critical for timely decision-making and effective monitoring of trial progress.
How do EDC platforms ensure participant safety?
High-quality information management is essential for protecting participant safety. EDC solutions adhere to regulations, such as the European Union Medical Device Regulation, which emphasizes participants' rights and well-being while ensuring the scientific validity of generated data.
What additional benefits do EDC systems offer?
EDC systems facilitate the integration of data from various sources, including electronic health records and real-world information, yielding valuable insights into participant health and factors influencing trial outcomes.
What is the significance of compliance with regulations like ISO 14155:2020?
Compliance with standards such as ISO 14155:2020 is crucial for managing medical information effectively and ensuring the safety and efficacy of medical devices utilized in trials.
How do EDC platforms contribute to reducing research costs?
By automating data collection and reducing reliance on manual entry, EDC systems minimize errors and speed up data processing, ultimately lowering research and development expenses.
What security measures do EDC solutions implement?
EDC platforms incorporate advanced encryption, strict access controls, and comprehensive audit trails to protect sensitive patient information, ensuring compliance with regulations like HIPAA and GDPR.
How do EDC systems enhance the integrity of clinical research?
By providing automatic data validation and reporting features, EDC systems help identify discrepancies and support informed decision-making, improving patient outcomes and advancing medical knowledge.
What future advancements can we expect from EDC platforms?
Future EDC platforms may incorporate artificial intelligence (AI) and machine learning (ML), improving data analysis and enabling real-time information collection through wearable devices and mobile apps, enhancing patient engagement and data insights.
What is the projected growth of Software as a Medical Device (SaMD)?
The market for SaMD is expected to grow at a compound annual growth rate of approximately 52% from 2023 to 2028, underscoring the increasing demand for reliable data management tools in clinical research.
Why is health data interoperability important?
Health data interoperability promotes standardization across platforms, facilitating reusable research capabilities and enhancing the validity and generalizability of clinical trial outcomes.