


Achieving ISO 13485 certification is critical for organizations aiming to excel in the Medtech landscape. This process encompasses several key steps, including:
By diligently following these steps, organizations not only ensure compliance with regulatory requirements but also significantly enhance operational efficiency, product quality, and market access. Ultimately, these improvements lead to increased customer satisfaction, underscoring the importance of this certification in fostering trust and reliability in medical devices.
Achieving ISO 13485 certification represents a critical milestone for organizations within the medical device industry, serving as a testament to their unwavering commitment to quality management and regulatory compliance. This globally recognized standard not only enhances product safety and efficacy but also unlocks new market opportunities and elevates operational efficiency. Nevertheless, the path to certification is laden with challenges—what essential steps and requirements must organizations navigate to ensure successful compliance?
The ISO 13485 certification is a globally acknowledged standard that outlines criteria for a quality management system (QMS) in the medical device sector. This standard emphasizes the critical importance of consistent design, development, production, installation, and servicing of medical devices. Understanding the ISO 13485 certification is essential for entities aiming to ensure product safety and efficacy while meeting regulatory requirements.
Key principles include:
These are vital components for maintaining high-quality standards throughout the product lifecycle. Familiarizing yourself with these foundational elements will prepare you for the subsequent steps toward certification.
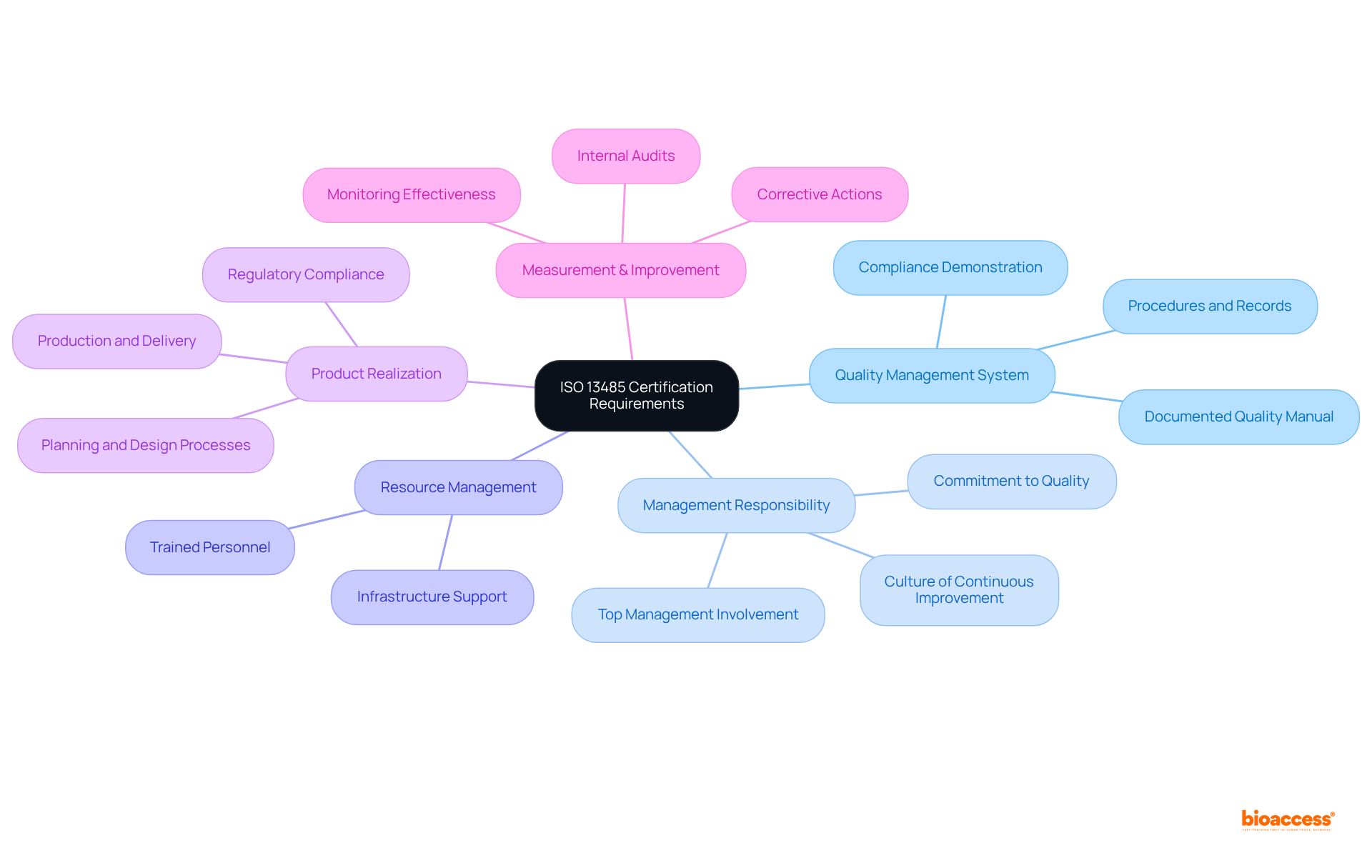
Organizations must meet several key requirements, including a documented quality management system, management responsibility, resource management, product realization, and measurement, analysis, and improvement to achieve ISO 13485 certification.
A documented quality management system (QMS) is essential; it requires the development and maintenance of a quality manual, procedures, and records. This documentation is vital for demonstrating compliance and operational consistency. Furthermore, top management must be actively involved in the QMS, showcasing a commitment to quality that fosters a culture prioritizing compliance and continuous improvement. Adequate resources, including trained personnel and infrastructure, must be allocated to support the QMS effectively.
Establishing processes for planning, design, development, production, and delivery of medical devices is crucial. These processes ensure that products consistently meet regulatory requirements and customer expectations. Additionally, organizations must implement processes for monitoring and measuring the effectiveness of the QMS, including internal audits and corrective actions that drive continuous improvement.
Grasping these requirements is essential for organizations to synchronize their practices with the ISO 13485 certification standards. The expense of ISO certification varies according to company size, typically ranging from $10,000 to more than $100,000. The certification process involves a two-stage audit, which is critical for compliance. Organizations that effectively establish a documented quality management system in accordance with ISO standards, such as achieving ISO 13485 certification, can enhance their credibility, operational efficiency, and market access. This ultimately results in increased customer satisfaction and a potential decrease in complaints by as much as 40%.
Moreover, ISO 13485:2016 aligns with ISO 9001:2008, providing a familiar framework for organizations experienced in quality management systems. Understanding and implementing these standards not only positions organizations for success but also reinforces their commitment to quality and continuous improvement.
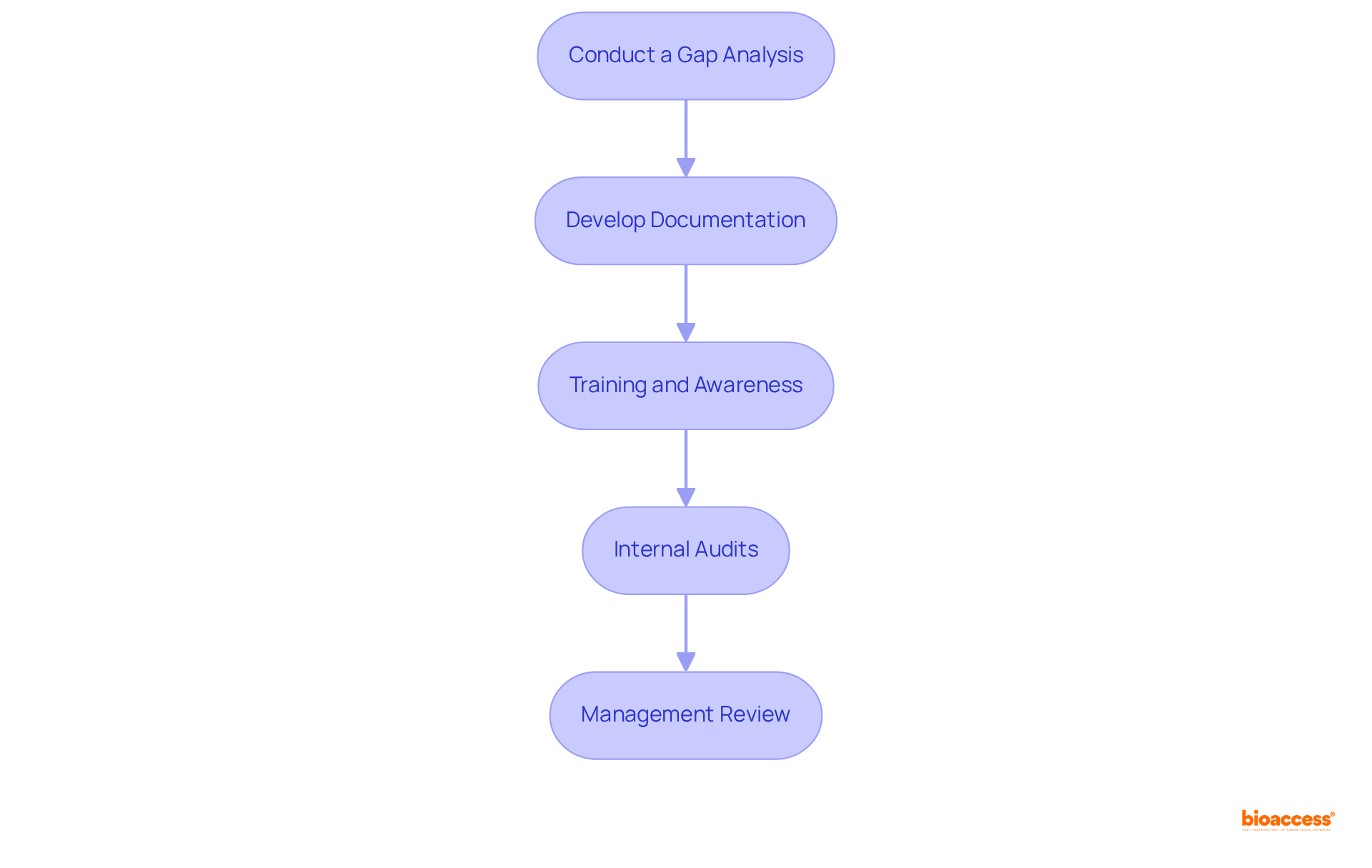
Implementing a Quality Management System (QMS) is essential for achieving ISO 13485 certification and involves several critical steps:
Conduct a Gap Analysis: Begin by evaluating your existing processes in relation to ISO standards. This analysis helps identify specific areas needing improvement, ensuring compliance with the standard. For optimal methods, consider utilizing resources from entities such as simpleQuE, which offers gap analysis services customized for ISO compliance.
Develop Documentation: Create essential documents, including a quality manual, procedures, and work instructions that accurately reflect your QMS. Proper documentation is crucial for demonstrating compliance with ISO 13485 certification during audits. As noted by Debbie Rivers Curry, the knowledge and guidance provided during this process can lead to obtaining ISO 13485 certification with zero non-conformities.
Training and Awareness: Provide comprehensive training for employees on the QMS and their specific roles within it. Ensuring that all team members understand their responsibilities is vital for the effective implementation of the system. Engaging training programs can significantly enhance employee awareness and compliance.
Internal Audits: Schedule regular internal audits to evaluate the effectiveness of the QMS. These audits help identify non-conformities and areas for improvement, fostering a culture of continuous enhancement. Companies that have outsourced internal audits often report smoother processes and better preparation for third-party audits.
Management Review: Conduct management reviews to assess the performance of the QMS. This step allows for necessary adjustments based on audit findings and overall system performance. Regular reviews ensure that the QMS remains aligned with organizational goals and regulatory requirements.
By diligently following these steps, companies can establish a strong QMS that not only meets ISO standards but also leads to ISO 13485 certification, enhancing operational efficiency and product quality. For instance, companies that have engaged in thorough gap analyses have reported smoother audit processes and higher success rates in maintaining their certifications. This proactive approach is essential for navigating the complexities of medical device regulations and ensuring patient safety. Furthermore, understanding the typical duration required to establish a QMS for ISO certification can assist in setting achievable expectations for your company.
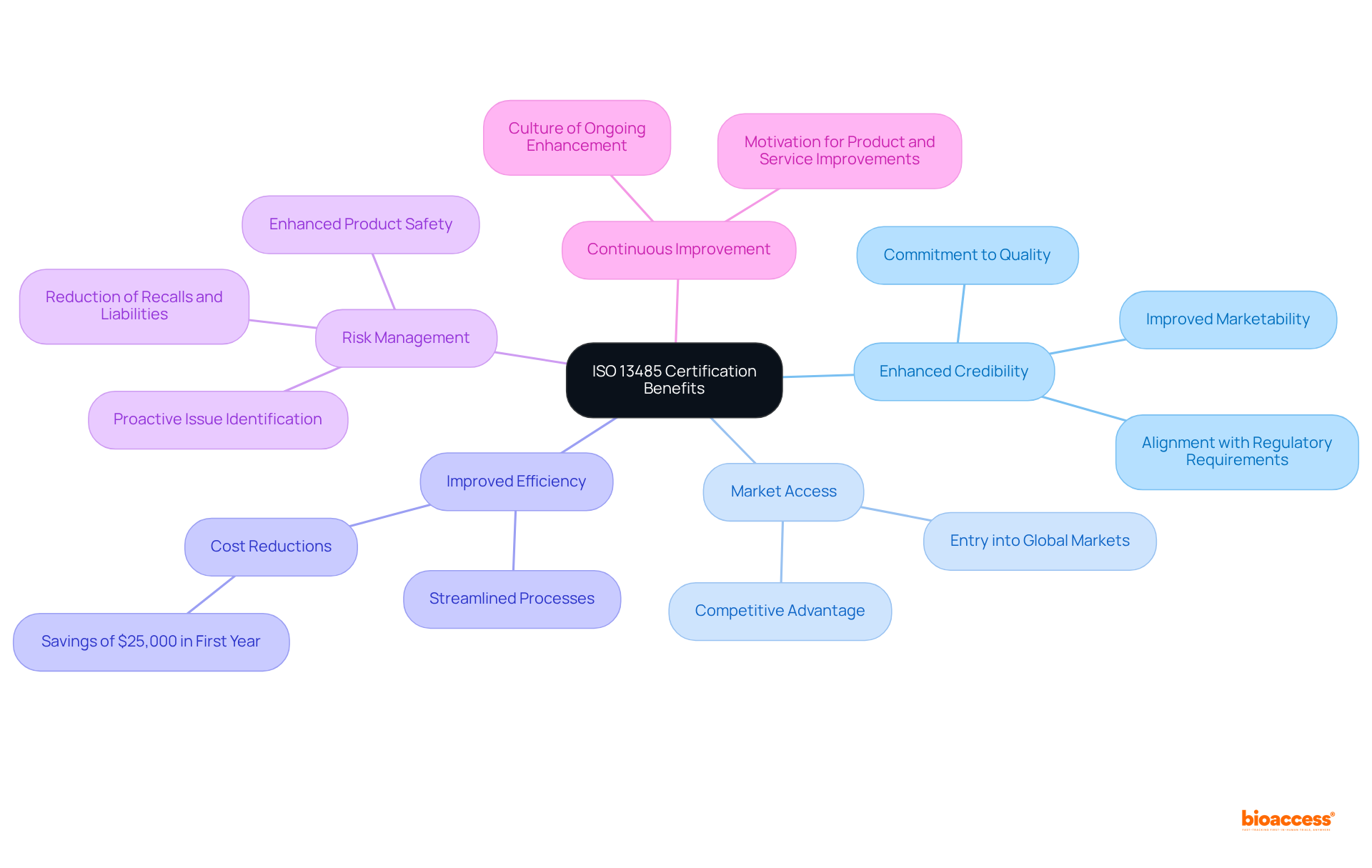
Organizations in the medical device sector can gain numerous benefits by achieving ISO 13485 certification.
Enhanced Credibility: Certification signifies a strong commitment to quality and compliance, fostering trust among customers and stakeholders. Organizations that obtain ISO certification can leverage this status to improve their marketability and credibility, as it aligns closely with regulatory requirements in various regions. Adhering to ISO 9001 standards greatly improves a company's credibility and market reputation.
Market Access: Numerous global markets require ISO certification for medical devices, making it an essential step for companies looking to broaden their reach. This certification not only opens doors to new regions but also positions organizations favorably in competitive landscapes, as compliance with such standards is often a prerequisite for entry.
Improved Efficiency: Implementing a Quality Management System (QMS) in line with ISO standards can streamline processes, reduce waste, and enhance overall operational efficiency. Organizations that adopt these practices frequently report considerable cost reductions, with studies showing that 67% of those implementing ISO 9001 achieve at least $25,000 in savings within the first year.
Risk Management: A core element of ISO 13485 is its focus on risk management, which allows entities to proactively identify and address potential issues before they escalate. This focus not only enhances product safety but also reduces the likelihood of costly recalls and legal liabilities, addressing the alarming statistic that defective medical devices cause approximately 200,000 injuries annually.
Continuous Improvement: The certification process fosters a culture of ongoing enhancement, motivating entities to consistently pursue improvements in their products and services. This principle is essential for maintaining high-quality standards and ensuring that companies remain competitive in an evolving market.
By understanding these benefits, organizations can better appreciate the value of pursuing ISO 13485 certification, which ultimately leads to improved product quality, enhanced customer satisfaction, and a stronger market position.
Achieving ISO 13485 certification stands as a pivotal milestone for organizations in the medical device industry, establishing a solid foundation for quality management systems (QMS). This certification not only guarantees compliance with regulatory standards but also significantly enhances product safety and efficacy. By grasping the essential principles and requirements of ISO 13485, organizations can adeptly navigate the complexities of certification, positioning themselves for success in an increasingly competitive market.
Key steps to attaining ISO 13485 certification encompass:
Each of these elements is crucial in establishing a QMS that adheres to ISO standards while fostering a culture of continuous improvement. Organizations that diligently follow these steps can anticipate substantial benefits, including enhanced credibility, improved operational efficiency, and greater market access.
Ultimately, pursuing ISO 13485 certification transcends mere compliance; it embodies a commitment to quality that can lead to heightened customer satisfaction and reduced operational risks. By embracing the principles of ISO 13485, organizations can ensure they remain competitive and responsive to the evolving demands of the medical device sector. Taking decisive action today to implement these practices can pave the way for a safer, more efficient future in medical device manufacturing.
What is ISO 13485 certification?
ISO 13485 certification is a globally recognized standard that outlines the criteria for a quality management system (QMS) specifically in the medical device sector.
Why is ISO 13485 certification important?
It is essential for ensuring product safety and efficacy while meeting regulatory requirements in the medical device industry.
What are the key principles of ISO 13485?
The key principles include a strong focus on risk management, process validation, and continuous improvement.
How does ISO 13485 contribute to product quality?
It emphasizes consistent design, development, production, installation, and servicing of medical devices, which are vital for maintaining high-quality standards throughout the product lifecycle.
What should entities do to prepare for ISO 13485 certification?
Familiarizing themselves with the foundational elements of the standard, including its key principles, is crucial for preparing for the certification process.