


To become a microbiologist in pharmaceuticals, one must master fundamental microbiology principles, pursue relevant education and certifications, gain practical experience, and build a professional network. This pathway is essential for anyone aiming to excel in the pharmaceutical industry.
Understanding microbial classification and pathogenesis is crucial, as it lays the groundwork for advanced study. Obtaining advanced degrees and certifications not only enhances knowledge but also significantly boosts career prospects.
Engaging in internships provides invaluable practical experience, while actively networking is vital for professional growth and opportunity.
These essential steps are designed to empower aspiring microbiologists, ensuring they are well-equipped to meet the challenges of the field.
The pharmaceutical industry stands at the forefront of medical innovation, propelled by the intricate world of microbiology. Aspiring microbiologists possess a unique opportunity to contribute to the development of life-saving drugs. However, navigating this complex field necessitates a strategic approach.
What essential steps must one take to transform a passion for microbiology into a successful career in pharmaceuticals, particularly in an era where antimicrobial resistance presents a critical challenge?
This guide explores the foundational knowledge, educational pathways, practical experiences, and networking strategies essential for anyone eager to make their mark in this dynamic sector.
To embark on your journey as a microbiologist in the drug industry, grasping the fundamental principles of microbiology is essential. This encompasses:
Microbial Classification: Understanding the various types of microorganisms—bacteria, fungi, viruses, and protozoa—and their specific roles in medical applications is crucial. Effective microbial classification aids in identifying pathogens and tailoring appropriate treatments, which is vital for drug development. For instance, the challenges posed by multidrug-resistant tuberculosis (MDR-TB) highlight the importance of accurate pathogen identification in developing effective therapies.
Microbial Pathogenesis: A thorough comprehension of how microorganisms cause diseases and their infection mechanisms is essential for creating effective medications. The damage-response framework illustrates the interaction between hosts and pathogens, emphasizing the need for innovative approaches in drug design. The emergence of multidrug-resistant organisms underscores the urgency for new antimicrobial agents, as the clinical pipeline for antibiotics is nearly depleted, with only 27 antibiotics in clinical development, of which only 6 are classified as innovative. Furthermore, antimicrobial resistance (AMR) contributed to approximately 4.95 million deaths in 2019, highlighting the critical need for effective drug development.
Antimicrobial Agents: Familiarity with various antimicrobial agents, their mechanisms of action, and their therapeutic applications is essential. The World Health Organization has emphasized the importance of effective antimicrobial stewardship to combat the rising threat of AMR.
Regulatory Standards: Examining the regulatory frameworks that oversee microbiological practices in drugs, such as Good Manufacturing Practices (GMP) and the role of the FDA, is essential. These standards ensure the safety and effectiveness of medicinal products, guiding the development process from research to market. WHO's guidance on monitoring resistance further emphasizes the significance of regulatory frameworks in ensuring the safety and effectiveness of medications.
By mastering these core principles, you will be well-prepared to navigate the complexities of microbial science as a microbiologist in the pharmaceutical industry, ultimately contributing to the advancement of effective therapeutic solutions.

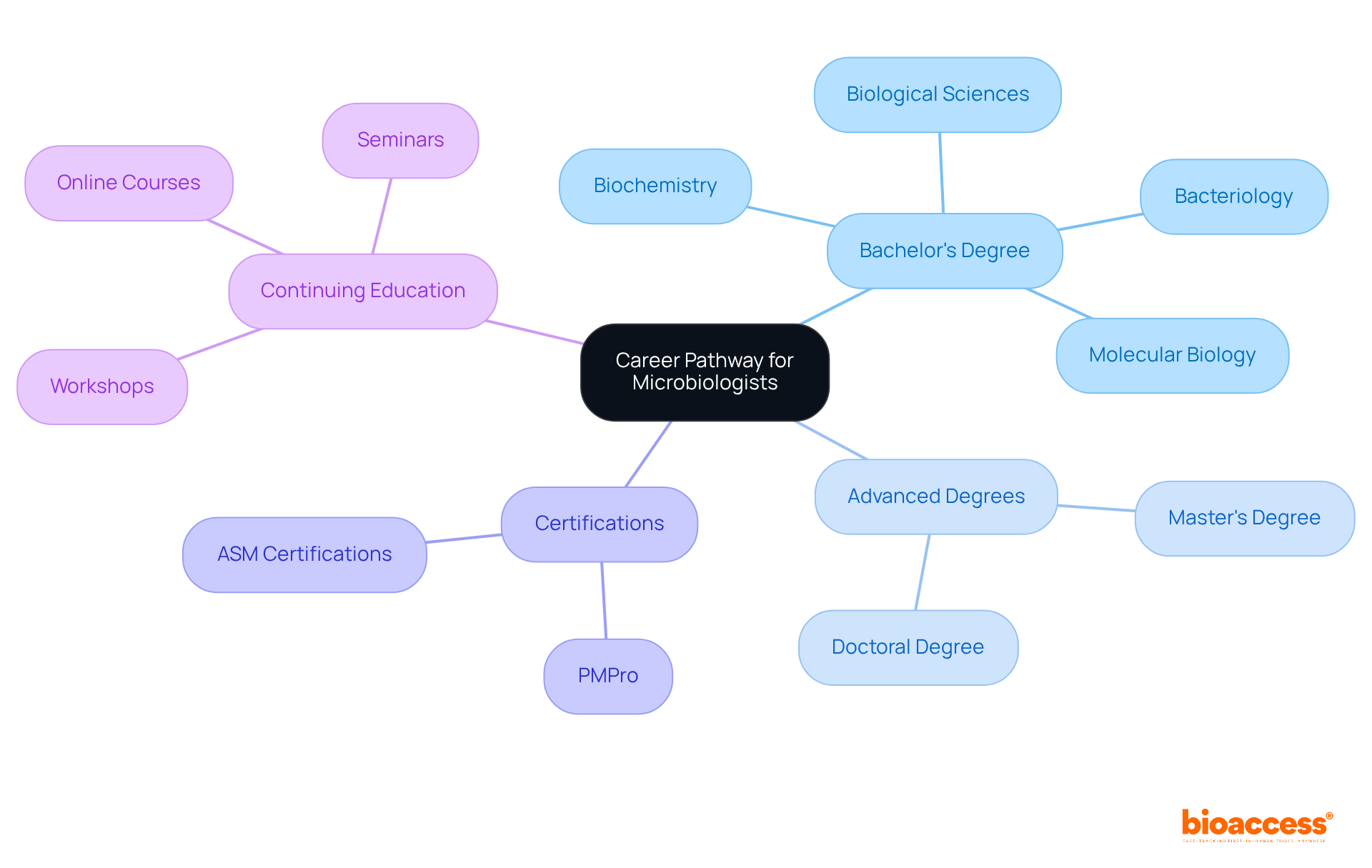
To establish a successful career as a microbiologist in the pharmaceutical industry, it is essential to follow a structured educational path. Begin with a Bachelor's Degree in biological sciences, biology, or a related field. Your coursework should encompass bacteriology, biochemistry, and molecular biology, laying a solid foundation for your future studies.
Next, consider pursuing Advanced Degrees such as a master's or doctoral degree in life sciences or drug development. This step will deepen your knowledge and enhance your research skills, positioning you as a competent professional in the field.
Additionally, obtaining Certifications such as the Pharmaceutical Microbiology Professional Certification (PMPro) or relevant certifications from the American Society for Microbiology (ASM) is crucial. These credentials will validate your expertise and demonstrate your commitment to the profession.
Finally, engage in Continuing Education through workshops, online courses, and seminars. This ongoing learning will keep you informed about the latest developments in drug-related sciences, ensuring you remain at the forefront of the industry.
By diligently following these educational pathways, you will construct a robust foundation for your career as a microbiologist in the pharmaceutical field.
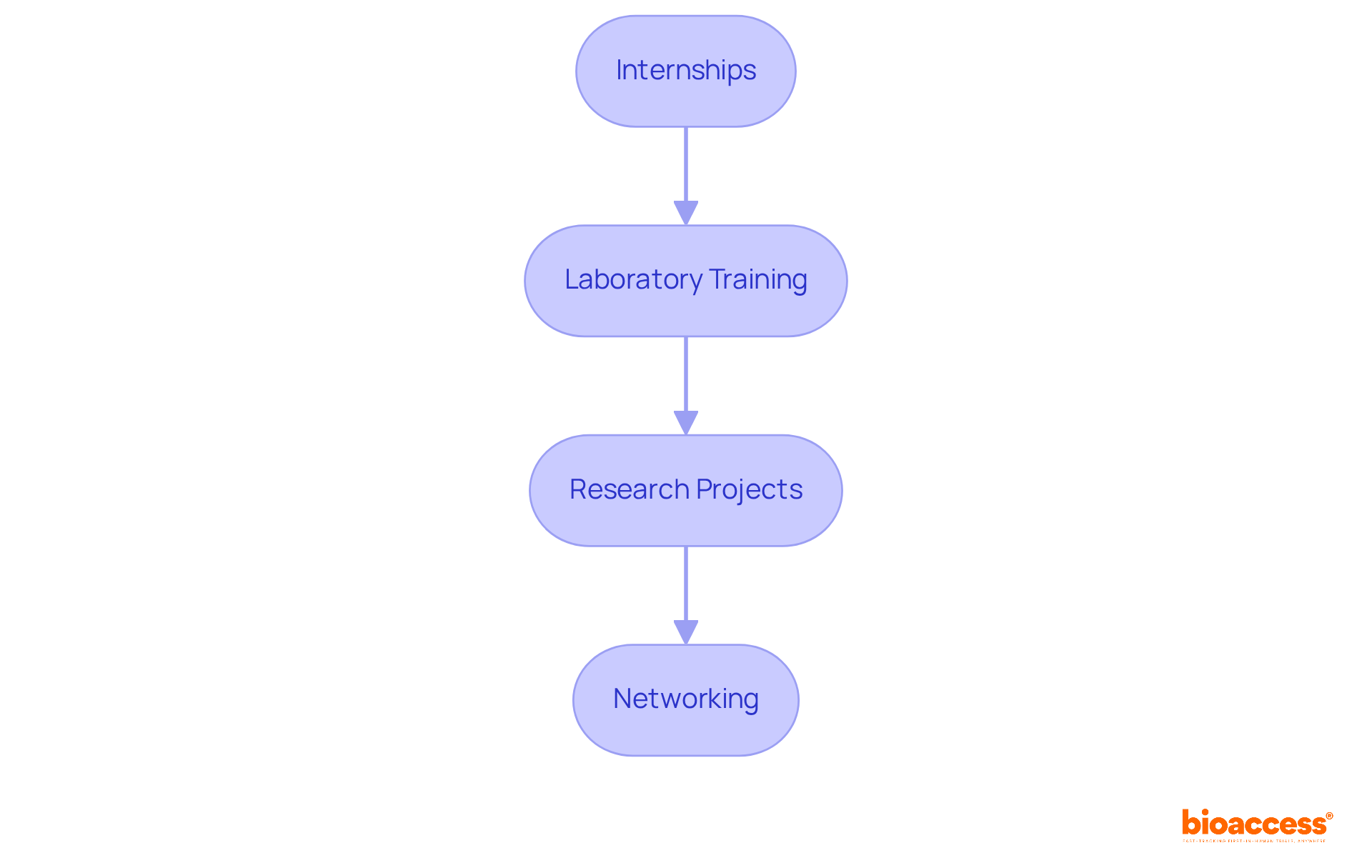
To gain practical experience in microbiology, consider the following steps:
By actively pursuing practical experiences, you will sharpen your skills and enhance your appeal to potential employers.
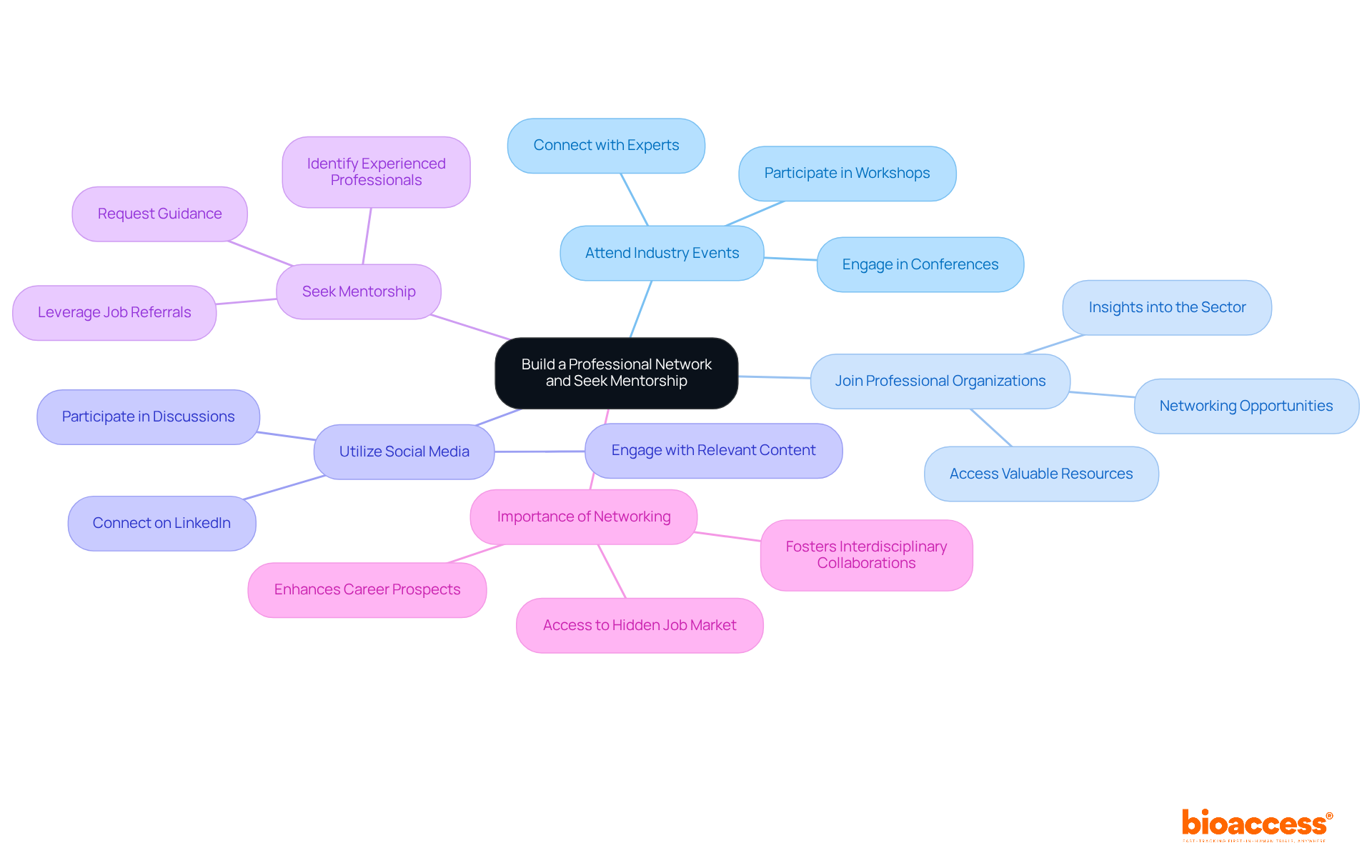
To effectively build your professional network and seek mentorship, consider the following strategies:
By actively networking and seeking mentorship, you will cultivate a support system that can greatly enhance your career prospects as a microbiologist in the pharmaceutical field.
To embark on a successful career as a microbiologist in the pharmaceutical industry, mastering the core principles of microbiology is essential. This involves:
Each of these steps is crucial in developing the knowledge and skills necessary to contribute effectively to drug development and address public health challenges.
Understanding microbial classification, pathogenesis, and regulatory standards is paramount. Advanced education and certifications further enhance one's expertise. Practical experiences, such as internships and laboratory training, are vital for applying theoretical knowledge in real-world settings. Moreover, building a professional network through industry events and mentorship can unlock career opportunities and provide invaluable insights for aspiring microbiologists.
Ultimately, the journey to becoming a microbiologist in pharmaceuticals demands dedication and a proactive approach to learning and networking. By following these essential steps, individuals can position themselves as competent professionals ready to confront the pressing challenges of antimicrobial resistance and contribute to the development of innovative therapeutic solutions. The significance of this field is profound, as it directly impacts public health and the effectiveness of medical treatments.
What fundamental principles of microbiology should one understand to work in the pharmaceutical industry?
Key principles include microbial classification, microbial pathogenesis, antimicrobial agents, and regulatory standards.
Why is microbial classification important in pharmaceuticals?
Microbial classification helps identify pathogens and tailor appropriate treatments, which is crucial for drug development, especially in cases like multidrug-resistant tuberculosis (MDR-TB).
What is microbial pathogenesis and its significance in drug development?
Microbial pathogenesis refers to how microorganisms cause diseases and their infection mechanisms. Understanding this is essential for creating effective medications and addressing the challenges posed by multidrug-resistant organisms.
What is the current state of antibiotic development in relation to antimicrobial resistance (AMR)?
The clinical pipeline for antibiotics is nearly depleted, with only 27 antibiotics in clinical development, and only 6 classified as innovative. AMR contributed to approximately 4.95 million deaths in 2019, highlighting the urgent need for new antimicrobial agents.
What role do antimicrobial agents play in pharmaceuticals?
Antimicrobial agents are critical for treating infections, and understanding their mechanisms of action and therapeutic applications is essential for effective drug development and combating AMR.
What regulatory standards are important for microbiological practices in drug development?
Good Manufacturing Practices (GMP) and the role of the FDA are key regulatory frameworks that ensure the safety and effectiveness of medicinal products throughout the development process.
How does the World Health Organization (WHO) contribute to antimicrobial stewardship?
The WHO emphasizes effective antimicrobial stewardship and provides guidance on monitoring resistance, which is vital for ensuring the safety and effectiveness of medications.