


The best practices for compliance in Mexico concerning Medtech and Biopharma are crucial for establishing a robust regulatory framework. These practices include:
Such measures are essential for navigating the intricate regulatory landscape, mitigating risks, and ensuring timely market entry. This is particularly significant in light of anticipated changes, including new Good Manufacturing Practices guidelines, which necessitate proactive adaptation and vigilance.
Navigating the intricate regulatory landscape of Mexico presents both opportunities and challenges for companies in the Medtech and Biopharma sectors.
With the Federal Commission for Protection against Sanitary Risks (COFEPRIS) at the helm, understanding the evolving compliance requirements is essential for success in this burgeoning market.
As organizations strive to align with new guidelines and enhance their operational frameworks, they must confront significant hurdles, including bureaucratic delays and complex documentation processes.
How can businesses effectively overcome these obstacles while ensuring adherence to best practices and maintaining a competitive edge?
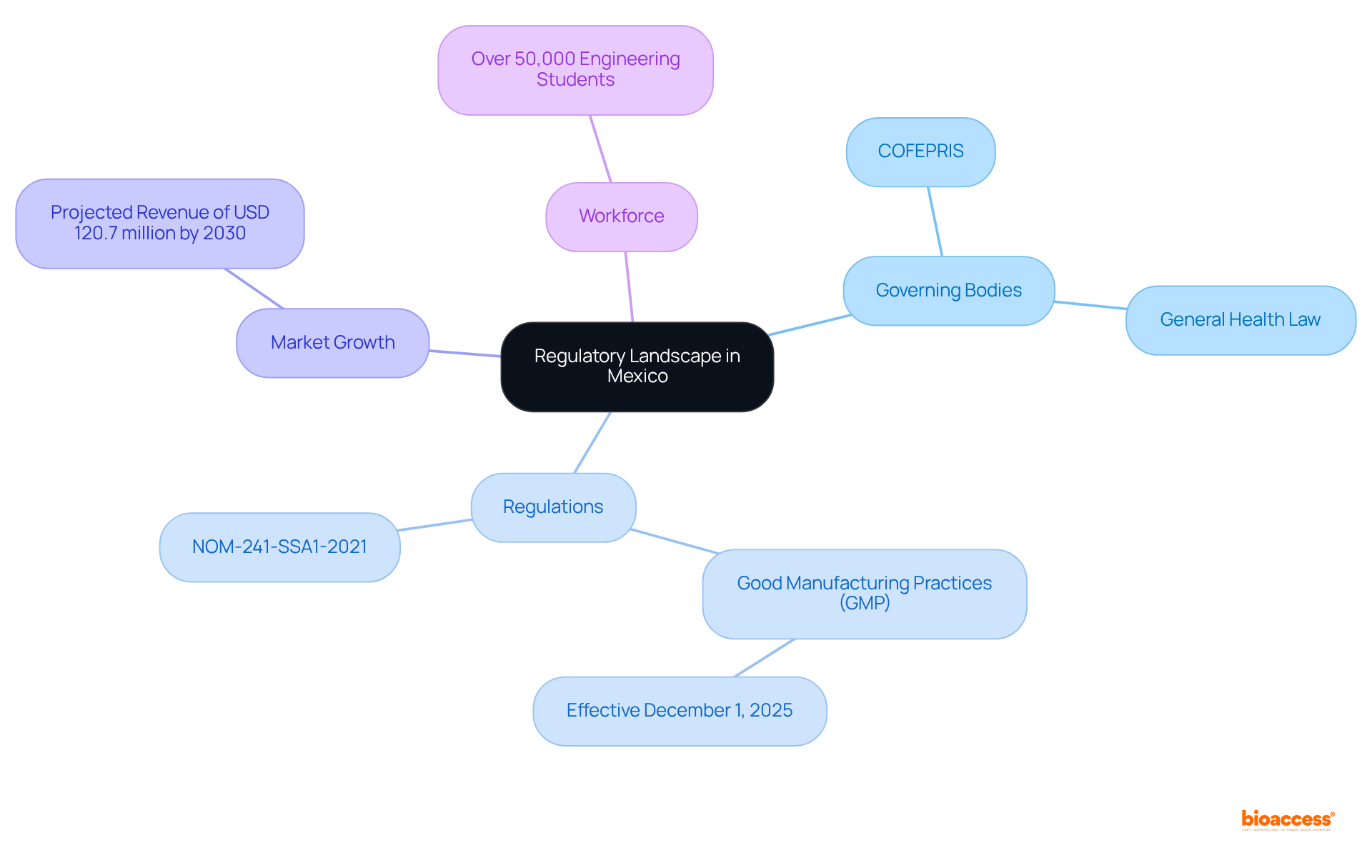
Effectively navigating the compliance landscape in Mexico requires a thorough understanding of the best practices for compliance in Mexico, as well as the key governing bodies and their requirements. The Federal Commission for Protection against Sanitary Risks (COFEPRIS) serves as the primary authority overseeing the approval of medical devices and pharmaceuticals. Familiarity with the General Health Law and specific regulations, such as NOM-241-SSA1-2021, is critical.
Companies must stay informed about recent changes, including the new Good Manufacturing Practices (GMP) guidelines set to take effect on December 1, 2025, which represent the best practices for compliance in Mexico to align the country's regulations with international standards. With over 90% of manufacturing plants in the region being ISO certified, companies can leverage this robust infrastructure to ensure the quality and safety of their products.
Furthermore, the medical device regulatory affairs market in Mexico is projected to reach a revenue of USD 120.7 million by 2030, underscoring the market's growth and significance. Engaging with regional specialists and legal consultants can provide essential insights into the intricacies of these regulations, helping to identify the best practices for compliance in Mexico and ensuring that all compliance measures are met.
Additionally, the area boasts a highly skilled workforce, with more than 50,000 engineering students trained in the medical device manufacturing sector, demonstrating its capacity to uphold regulatory and quality standards efficiently.
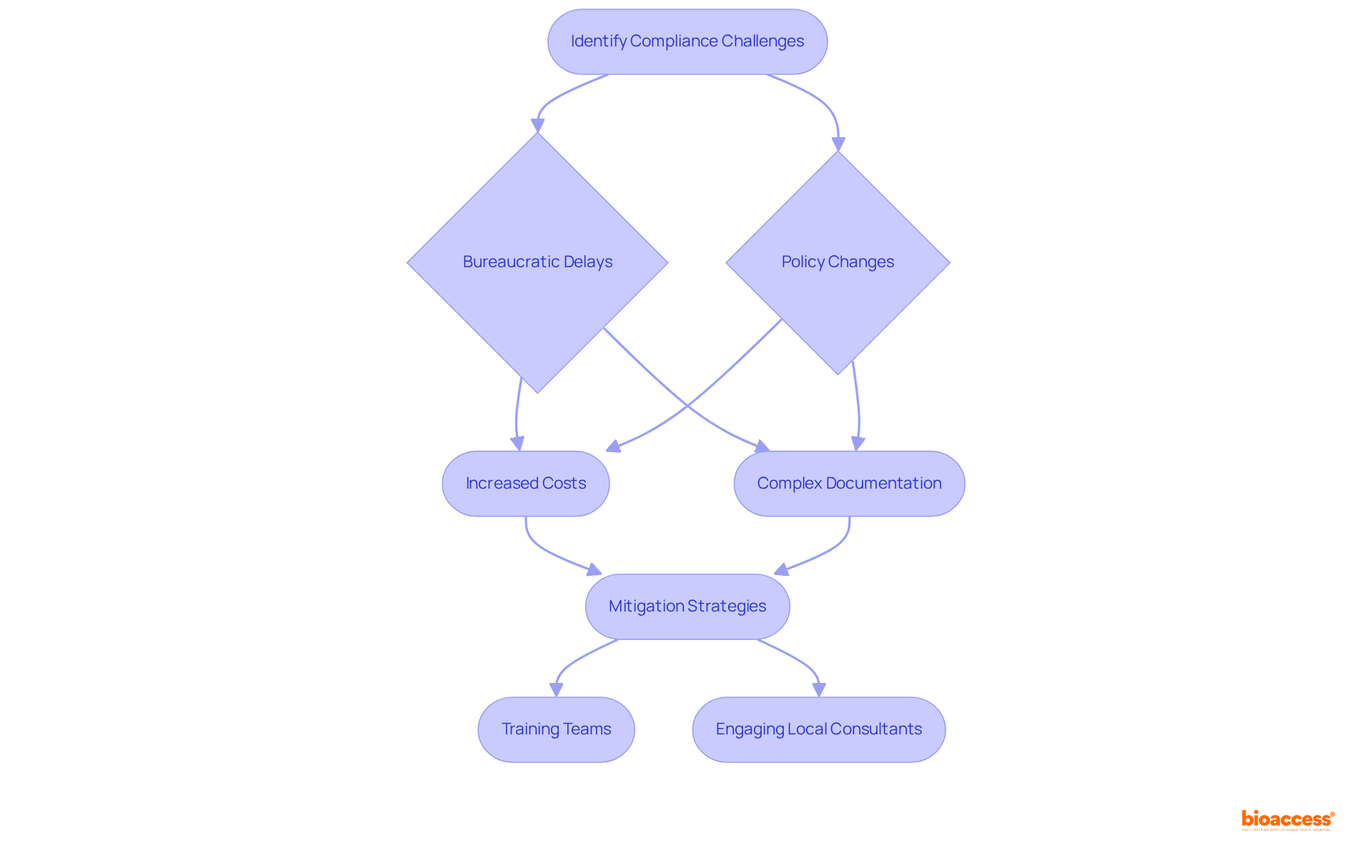
Navigating compliance in Mexico's Medtech and Biopharma sectors poses significant challenges, particularly due to bureaucratic delays at COFEPRIS. These delays can extend approval timelines, resulting in increased costs and hindering timely market entry. For instance, while the average duration for generic medicine approval has improved from 36 to 24 months, many firms still face a backlog of incomplete procedures, complicating the approval process. With a population of 126 million, the impact of these delays is profound, as they can affect access to essential medical products. Moreover, frequent policy changes and the necessity for thorough documentation introduce layers of complexity that can overwhelm organizations. Language barriers and cultural differences further complicate communication with governing authorities, making it essential for companies to adapt effectively.
To mitigate these challenges, organizations should prioritize:
As Juan Luis Serrano Leets highlighted, a significant challenge for COFEPRIS is enhancing the efficiency and speed of its regulatory approval processes. Establishing early communication with the agency is crucial to ensure we follow the best practices for compliance in Mexico regarding submission requirements and effectively navigate the intricacies of the approval process. Furthermore, organizations should be cautious not to undervalue the complexity of COFEPRIS interactions or to engage local consultants ineffectively. By adopting these strategies, including leveraging bioaccess®'s capability to deliver ethical approvals in 4-6 weeks, companies can better position themselves to overcome bureaucratic hurdles and accelerate their path to market.
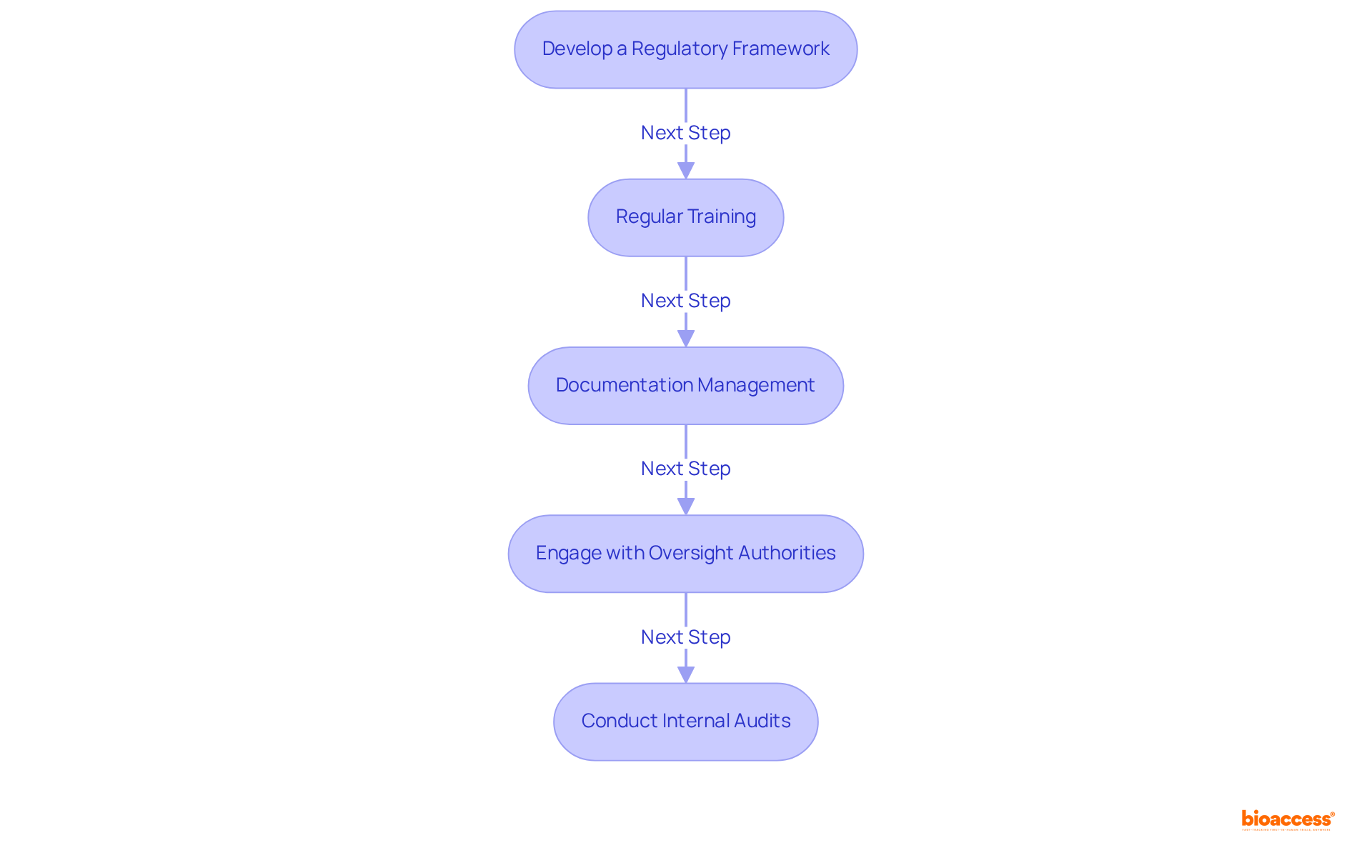
To ensure regulatory compliance in Mexico, organizations must adopt several best practices:
Develop a Regulatory Framework: Establish a comprehensive regulatory framework that clearly outlines roles, responsibilities, and procedures for adherence to regulations. This framework serves as the foundation for consistent adherence efforts throughout the organization. Recent findings indicate that 60% of executives report increased investment in regulatory resources, underscoring the growing recognition of its importance.
Regular Training: Conduct ongoing training sessions for staff on the latest regulations and adherence requirements. Keeping everyone informed is essential for fostering a culture of adherence and ensuring that all team members comprehend their responsibilities. Engaging, role-specific training can significantly enhance the adherence culture.
Documentation Management: Implement a robust documentation management system to ensure that all necessary documents are readily available and up-to-date. Effective documentation is crucial for demonstrating adherence during audits and inspections. As noted, effective compliance management strengthens internal processes, earns stakeholder trust, and enhances resilience.
Engage with Oversight Authorities: Foster open communication with COFEPRIS and other oversight bodies. Proactively clarifying requirements and addressing concerns can help organizations navigate the regulatory landscape more effectively. This engagement can prevent common pitfalls, such as inconsistent or undocumented controls, which many organizations face.
Conduct Internal Audits: Regularly perform internal audits to identify adherence gaps and areas for improvement. This proactive approach ensures that corrective actions are taken promptly, minimizing the risk of non-compliance and associated penalties. Organizations that implement these practices can anticipate lowering regulatory expenses while increasing organizational trust.
By incorporating best practices for compliance in Mexico, organizations can enhance their regulatory stance, reduce risks, and facilitate the successful advancement of medical innovations in the Mexican market.
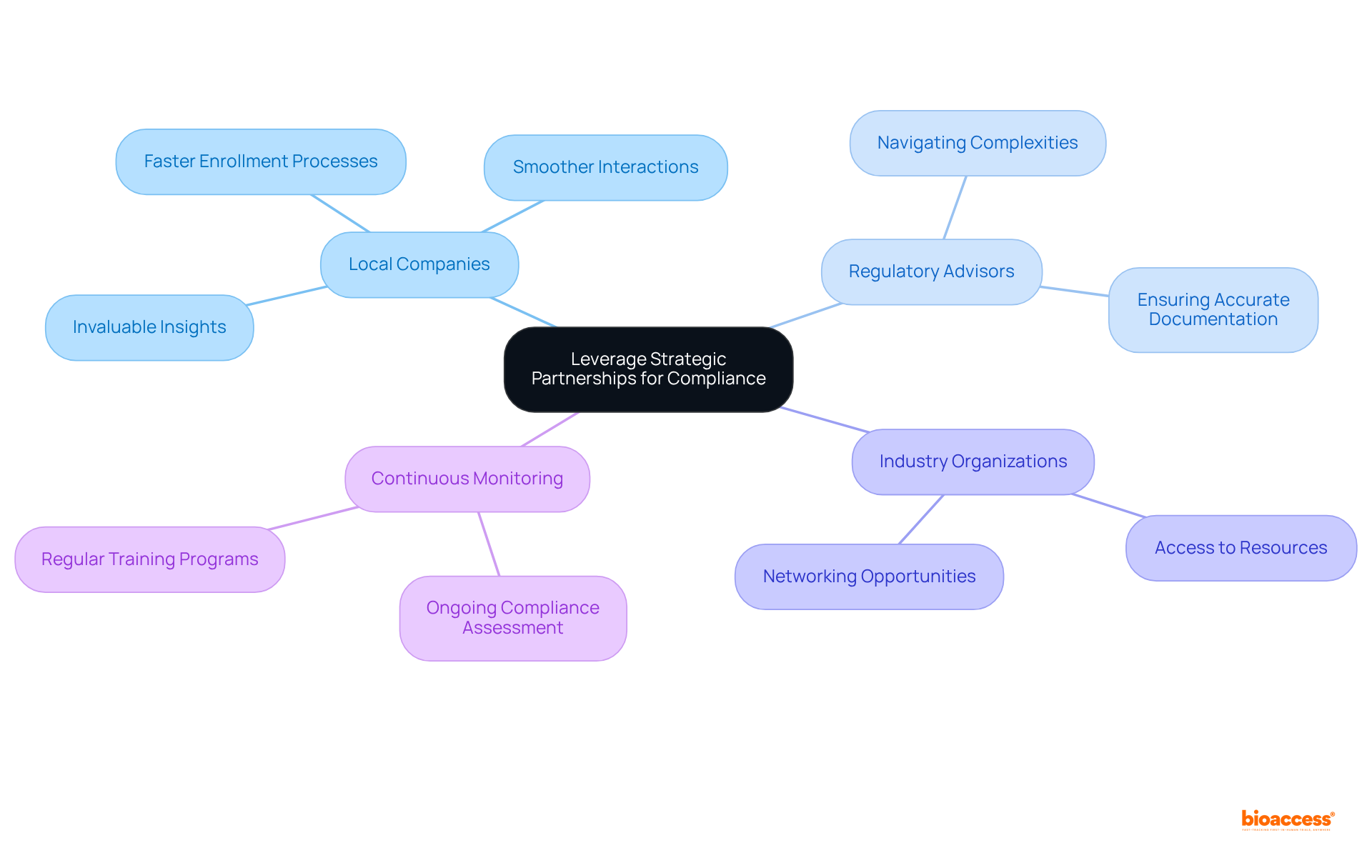
Utilizing strategic alliances significantly enhances the best practices for compliance in Mexico. Organizations should consider partnering with local companies that have established connections with oversight entities, such as COFEPRIS, as these partnerships can provide invaluable insights into the best practices for compliance in Mexico and facilitate smoother interactions.
These local partners possess a deep understanding of the legal framework, which helps companies to follow best practices for compliance in Mexico and navigate requirements more efficiently. Additionally, collaborating with regulatory advisors can aid in navigating the complexities of compliance requirements and help ensure adherence to the best practices for compliance in Mexico, making certain that all documentation is accurate and comprehensive.
Engaging with industry organizations can also provide access to resources, training, and networking opportunities that further support the best practices for compliance in Mexico. Continuous monitoring of compliance efforts is crucial to ensure ongoing adherence to regulations.
By cultivating a network of reliable partners, organizations can enhance their compliance capabilities and apply best practices for compliance in Mexico to mitigate the risks associated with regulatory non-conformity. For instance, businesses that collaborate with regional firms can benefit from expedited enrollment processes, evidenced by the fact that enrollment is 50% faster than in conventional markets.
Moreover, the experiences of organizations like Qually highlight the challenges faced in the clinical research sector, underscoring the importance of local partnerships in overcoming compliance obstacles. Ultimately, leveraging strategic partnerships is essential for implementing best practices for compliance in Mexico, as it not only reduces risks but also positions companies for long-term success in the competitive Medtech and Biopharma landscape.
Understanding the intricate regulatory environment in Mexico is essential for companies in the Medtech and Biopharma sectors. Familiarizing themselves with the requirements set forth by COFEPRIS and the General Health Law enables organizations to navigate compliance challenges more effectively. The anticipated changes in Good Manufacturing Practices (GMP) and the projected growth of the medical device regulatory market underscore the importance of remaining informed and proactive in compliance efforts.
Key strategies include:
Organizations should prioritize open communication with oversight bodies and undertake regular internal audits to identify and address compliance gaps. Furthermore, leveraging strategic partnerships can significantly streamline the compliance process, allowing companies to navigate the complexities of the regulatory landscape more efficiently.
As the Medtech and Biopharma markets in Mexico continue to evolve, the significance of implementing best practices for compliance cannot be overstated. Companies are encouraged to adopt these strategies not only to mitigate risks but also to position themselves for sustainable success in a competitive environment. Embracing compliance as a cornerstone of operations will ultimately lead to improved product access and better healthcare outcomes for the population.
What is the primary authority overseeing the approval of medical devices and pharmaceuticals in Mexico?
The primary authority is the Federal Commission for Protection against Sanitary Risks (COFEPRIS).
Why is familiarity with the General Health Law important for companies in Mexico?
Familiarity with the General Health Law and specific regulations is critical for navigating the compliance landscape and ensuring adherence to legal requirements.
What are the new Good Manufacturing Practices (GMP) guidelines, and when do they take effect?
The new Good Manufacturing Practices (GMP) guidelines represent best practices for compliance in Mexico and are set to take effect on December 1, 2025.
What percentage of manufacturing plants in Mexico are ISO certified?
Over 90% of manufacturing plants in the region are ISO certified.
What is the projected revenue for the medical device regulatory affairs market in Mexico by 2030?
The projected revenue for the medical device regulatory affairs market in Mexico is USD 120.7 million by 2030.
How can companies ensure they meet compliance measures in Mexico?
Companies can engage with regional specialists and legal consultants to gain insights into regulations and identify best practices for compliance.
What is the significance of the workforce in Mexico's medical device manufacturing sector?
The area boasts a highly skilled workforce, with more than 50,000 engineering students trained in the medical device manufacturing sector, demonstrating its capacity to uphold regulatory and quality standards efficiently.