


Ensuring pharmaceutical data integrity in research is paramount. Best practices include:
These practices are essential for achieving accuracy, reliability, and compliance with regulatory standards. By adhering to these guidelines, researchers can produce credible outcomes that safeguard patient safety and enhance the validity of clinical trial results. Ultimately, the commitment to data integrity not only fosters trust in research but also contributes to the overall advancement of clinical practices.
Pharmaceutical data integrity serves as a cornerstone of clinical research, significantly influencing both the validity of study outcomes and the safety of involved patients. As the demand for reliable and compliant data escalates, it is crucial for researchers and organizations to understand the best practices for maintaining this integrity. Yet, amidst evolving regulatory landscapes and intricate data management processes, how can researchers guarantee that their data remains accurate, consistent, and trustworthy? This article explores the essential principles and strategies that underpin pharmaceutical data integrity, providing insights that can bolster the credibility of clinical trials and facilitate informed decision-making.
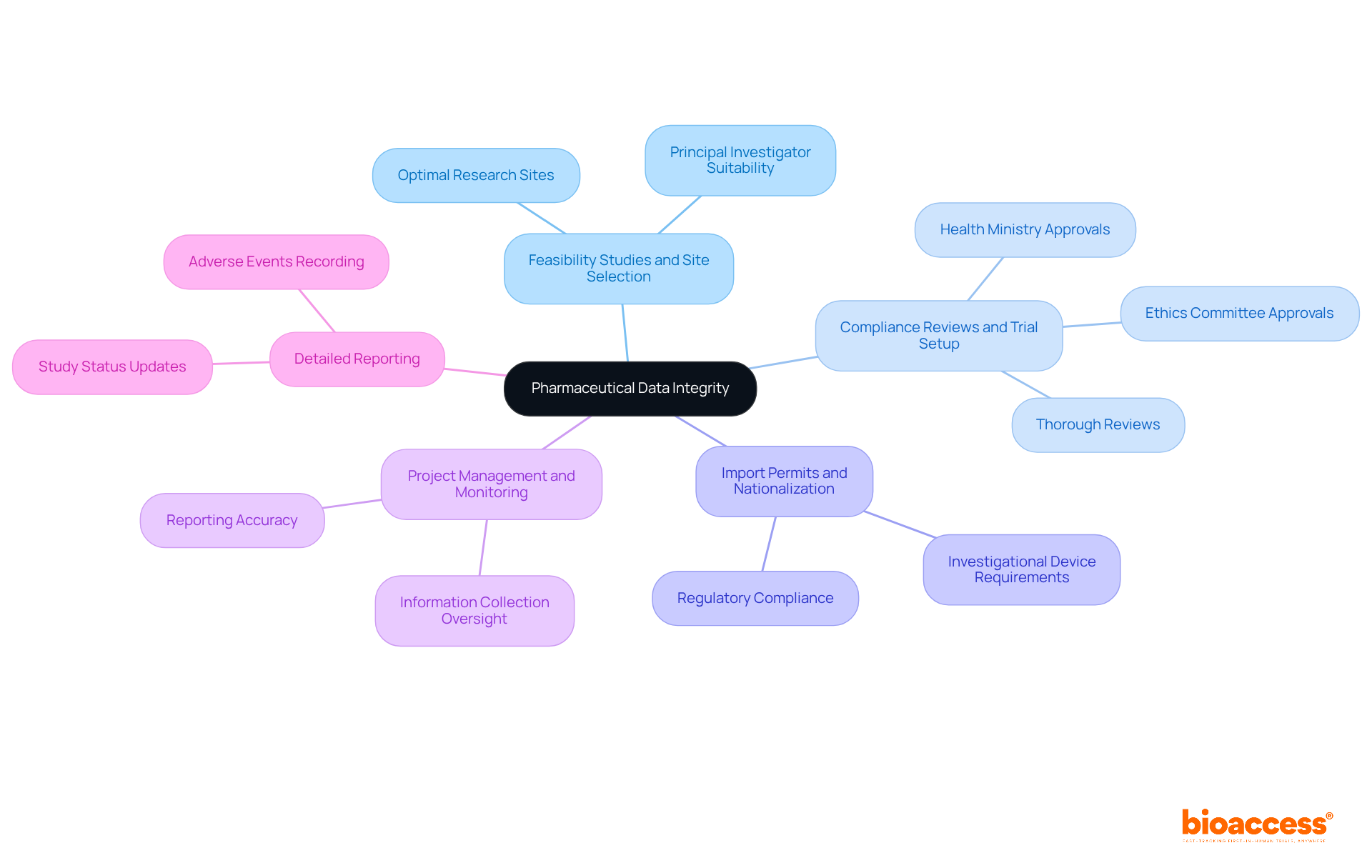
Pharmaceutical data integrity reflects the precision, consistency, and reliability of information throughout its lifecycle in clinical research. This concept encompasses all aspects of information gathering, processing, and reporting, ensuring the pharmaceutical data integrity is complete, reliable, and compliant with regulatory standards. Data reliability is paramount in clinical trials, as it directly impacts the pharmaceutical data integrity, the validity of study results, and the safety of patients involved. At bioaccess, we uphold data integrity through a comprehensive suite of clinical trial management services:
In essence, preserving pharmaceutical data integrity involves ensuring that the information accuracy reflects the true results of the research, free from manipulation or error. This commitment is critical in supporting informed decision-making within the context of pharmaceutical data integrity.
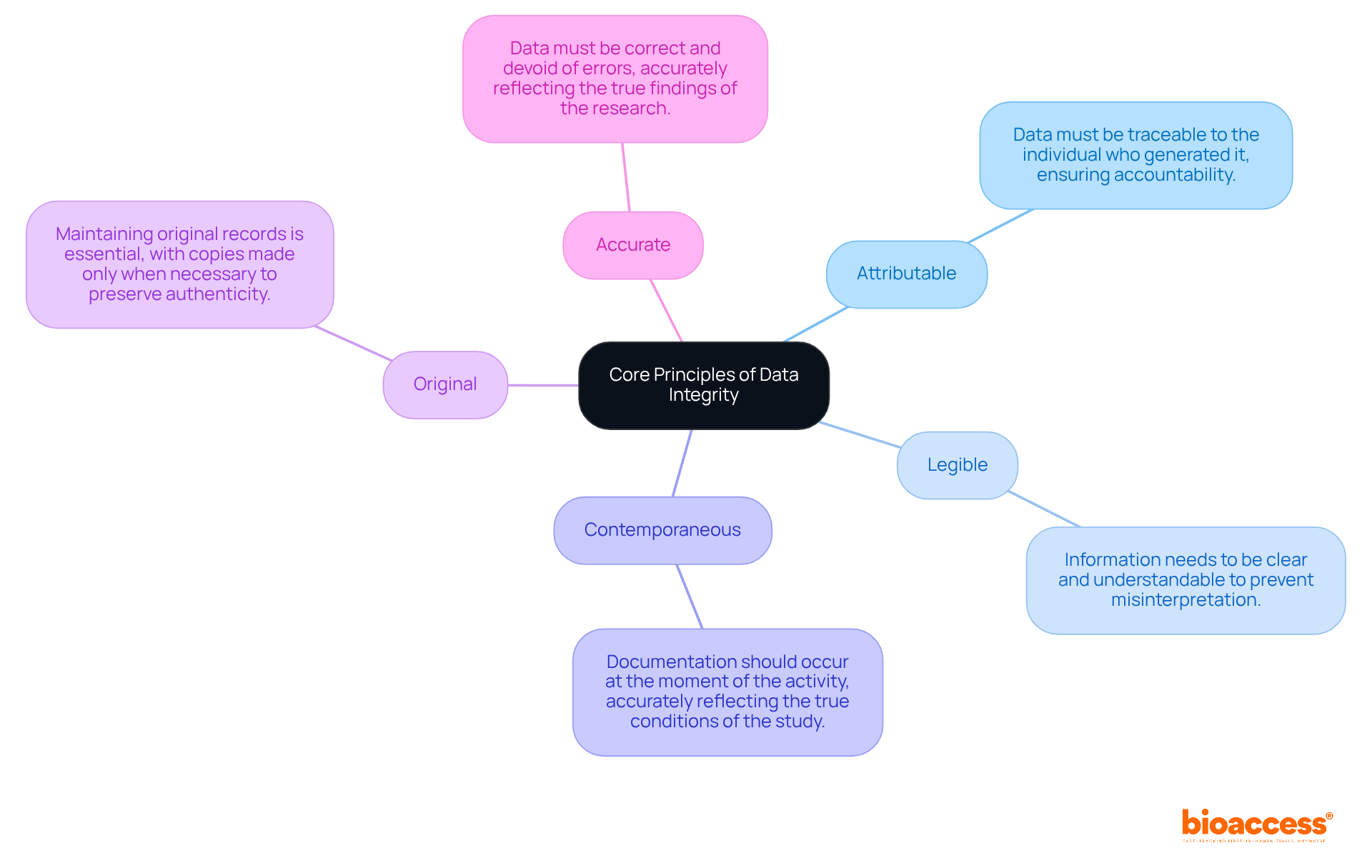
The fundamental concepts of information accuracy are encapsulated in the acronym ALCOA, which stands for Attributable, Legible, Contemporaneous, Original, and Accurate. Each principle is crucial for ensuring data integrity in clinical research.
By adhering to these principles, researchers can enhance the reliability of their findings and foster trust among stakeholders.
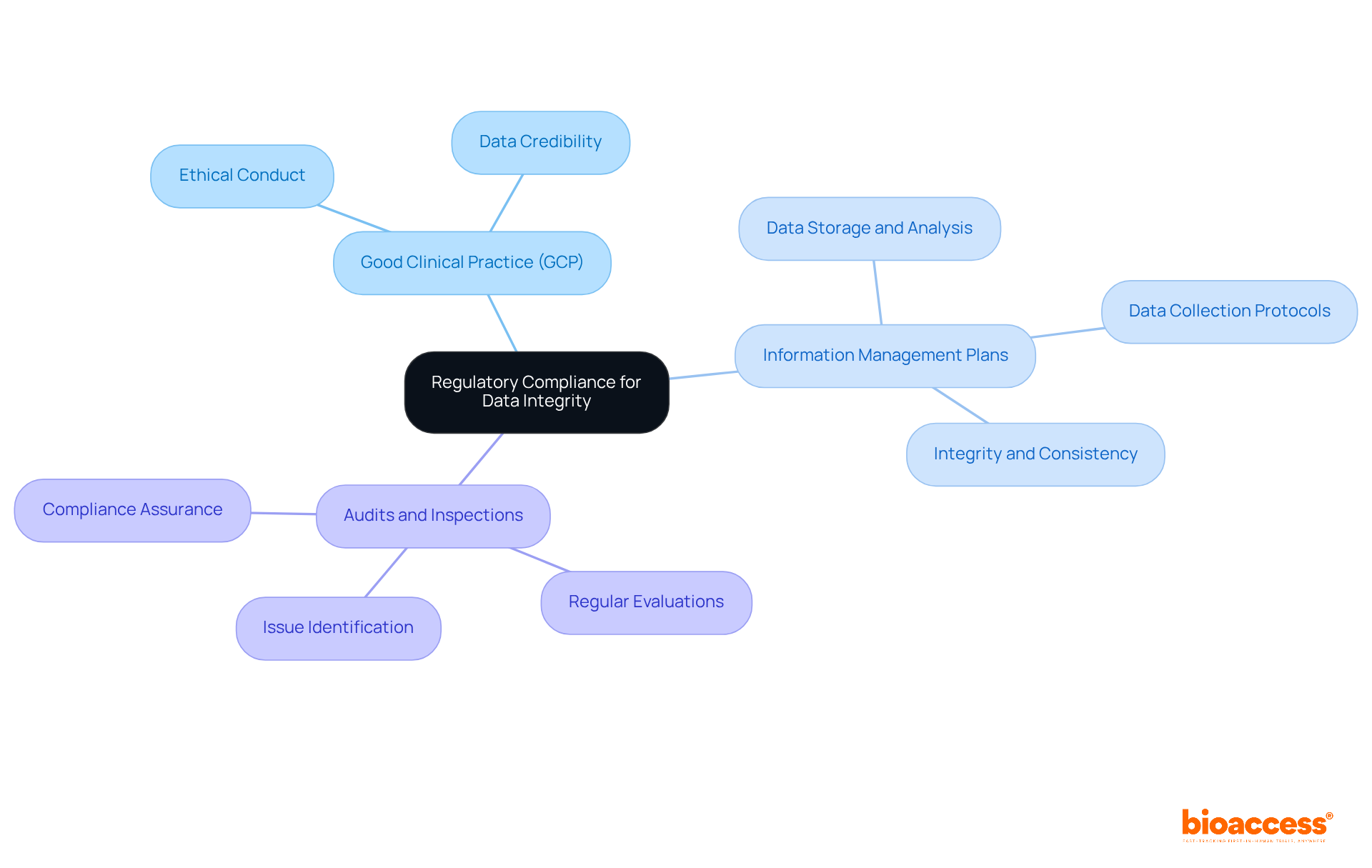
Regulatory compliance for pharmaceutical data integrity is crucial in clinical research, necessitating adherence to guidelines set forth by organizations such as the FDA, EMA, and ICH. These regulations delineate expectations for information management, documentation, and reporting in clinical trials. Key aspects of compliance include:
bioaccess® provides solutions that accelerate site activation and deliver FDA/EMA/MDR-ready datasets in under 8 weeks. With centralized monitoring and parallel submissions across LATAM, the Balkans, and Australia, bioaccess® is positioned to address key challenges in the Medtech landscape. By understanding and implementing these compliance measures, researchers can safeguard pharmaceutical data integrity and enhance the overall quality of their studies.
To maintain pharmaceutical data integrity in research, it is crucial to adopt best practices that ensure reliability and compliance. First, Training and Education is essential; all team members must be educated on information integrity principles and regulatory requirements, fostering a culture of compliance. Next, the establishment of Standard Operating Procedures (SOPs) is vital. Creating and executing SOPs for information collection, entry, and management guarantees consistency and precision. Additionally, Information Verification should be a regular practice, confirming information through checks and audits to swiftly identify and correct errors. Moreover, Secure Information Storage is imperative; utilizing secure systems for information storage and access control prevents unauthorized alterations. Finally, maintaining comprehensive Documentation of all study activities, including information collection techniques and modifications made, provides a clear audit trail. By implementing these practices, researchers can significantly enhance pharmaceutical data integrity, ultimately leading to more reliable and credible research outcomes.

Pharmaceutical data integrity stands as a cornerstone of credible clinical research, ensuring that the information collected is accurate, reliable, and compliant with regulatory standards. By maintaining data integrity, researchers uphold the validity of their findings while protecting the safety and well-being of participants involved in clinical trials. This unwavering commitment to data integrity is essential for fostering trust among stakeholders and supporting informed decision-making within the pharmaceutical industry.
Key insights from the article underscore the importance of adhering to the ALCOA principles—Attributable, Legible, Contemporaneous, Original, and Accurate—alongside a thorough understanding of regulatory compliance as dictated by organizations like the FDA and EMA. Implementing best practices, such as:
enhances the reliability of research outcomes. Through these measures, researchers can significantly bolster the integrity of their data, ultimately leading to more trustworthy results.
The significance of pharmaceutical data integrity cannot be overstated; it serves as the foundation for ethical research practices and the advancement of medical knowledge. As the landscape of clinical research continues to evolve, prioritizing data integrity will be vital for ensuring that studies yield credible results that effectively inform healthcare decisions. Embracing these best practices transcends mere regulatory requirements; it embodies a commitment to excellence in research that benefits the entire medical community.
What is pharmaceutical data integrity?
Pharmaceutical data integrity refers to the precision, consistency, and reliability of information throughout its lifecycle in clinical research, ensuring that the data is complete, reliable, and compliant with regulatory standards.
Why is data reliability important in clinical trials?
Data reliability is crucial in clinical trials because it directly impacts pharmaceutical data integrity, the validity of study results, and the safety of patients involved.
What services does bioaccess provide to uphold data integrity?
Bioaccess upholds data integrity through a comprehensive suite of clinical trial management services, including feasibility studies and site selection, compliance reviews and trial setup, import permits and nationalization of investigational devices, project management and monitoring, and detailed reporting.
How does bioaccess ensure site selection for clinical trials?
Bioaccess ensures that research sites and principal investigators are optimally suited for the trials through feasibility studies and site selection.
What steps does bioaccess take for compliance in clinical trials?
Bioaccess conducts thorough compliance reviews and secures necessary approvals from ethics committees and health ministries during trial setup.
What is involved in project management and monitoring by bioaccess?
Project management and monitoring by bioaccess involve overseeing the study to guarantee precise information collection and reporting.
How does bioaccess handle reporting during clinical trials?
Bioaccess provides updates on study status and meticulously records adverse events to accurately represent the study's outcomes.
What is the ultimate goal of preserving pharmaceutical data integrity?
The ultimate goal of preserving pharmaceutical data integrity is to ensure that the accuracy of information reflects the true results of the research, free from manipulation or error, supporting informed decision-making.