Overview
Best practices for medical device trials are centered on the adherence to principles such as:
- Good Clinical Practice
- Regulatory compliance
- Risk management
- Effective information management
These elements are crucial for ensuring safety and efficacy in clinical research. Integrating these principles not only enhances trial quality and participant safety but also facilitates smoother regulatory processes. Furthermore, this integration significantly improves overall outcomes in medical technology development, underscoring the importance of a structured approach in navigating the complexities of clinical trials.
Introduction
In the dynamic field of medical device trials, grasping the foundational principles and best practices is imperative for guaranteeing the safety and efficacy of new technologies. The clinical research landscape is continually evolving, shaped by regulatory frameworks, technological advancements, and diverse patient populations. As organizations endeavor to navigate these complexities, strict adherence to guidelines such as Good Clinical Practice (GCP) and ISO 14155:2020 becomes essential. The integration of robust methodologies, effective data management, and proactive risk assessment not only enhances trial outcomes but also cultivates trust among stakeholders.
This article investigates the key elements that define successful medical device trials, examining the challenges and opportunities within the Latin American context, and highlighting the innovative strategies poised to propel the industry forward.
Fundamentals of Medical Device Trials: Key Principles and Practices
Medical device evaluations are governed by essential principles that ensure the safety and efficacy of the devices being tested. These principles are foundational to the success of clinical research and include:
- Good Clinical Practice (GCP): Adhering to GCP guidelines is paramount for maintaining the integrity of clinical trials. This includes ensuring informed consent, protecting participant rights, and safeguarding information integrity. In 2025, adherence to GCP remains a critical focus, with recent statistics indicating that 45% of information from leading organizations is entered on the same day as the visit date. This statistic highlights the dedication to prompt and precise data management, which is essential for preserving data integrity in research studies.
- Regulatory Compliance: A thorough understanding of local and international regulations is crucial for the successful execution of the study. Familiarity with the requirements set forth by regulatory bodies such as the FDA and EMA is essential. In Colombia, for instance, the INVIMA (National Food and Drug Surveillance Institute) plays a pivotal role in overseeing medical device regulations, classified as a Level 4 health authority by PAHO/WHO. The effect of regulatory compliance on clinical studies outcomes cannot be overstated; it directly influences the approval process and the overall success of medical devices in the market. The era of EHR-facilitated PCTs offers a chance for reappraisal and redesign of research conduct, enhancing regulatory compliance and data management practices.
- Risk Management: Identifying and mitigating risks associated with studies is vital for participant safety and study integrity. Conducting comprehensive risk assessments and implementing strategies to minimize potential adverse effects are key components of effective risk management. Recent case studies emphasize the significance of proactive risk management in improving outcomes and participant safety. Utilizing registries, like SWEDEHEART for cardiac patients, can offer valuable information for risk management and enhance the overall quality of clinical studies.
- Information Management: Efficient information gathering and management methods are essential to guarantee the dependability of test outcomes. Employing validated data collection tools and maintaining precise records throughout the process are critical. Innovative experimental designs, like those observed in the TASTE and SAFE-PCI projects, illustrate the benefit of utilizing existing registries for patient enrollment and endpoint collection, improving the relevance of results to real-world medical practice.
Integrating these principles into the design and implementation of medical device studies not only aligns with the best practices for medical device trials but also promotes a culture of excellence in research. As Ashley Davidson, vice president of product lead - sponsor tech strategy, noted, "We need more site-centric approaches in project startup," emphasizing the importance of participant engagement and site management. Additionally, extensive management services provided by bioaccess, encompassing feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, enable the successful execution of studies.
As the landscape of research trials evolves, embracing these foundational elements will be essential for advancing medical technology and improving patient outcomes, while also contributing to local economies through job creation and healthcare improvement.

Navigating Regulatory Frameworks: Understanding ISO 14155:2020
ISO 14155:2020 outlines essential requirements and best practices for medical device trials, encompassing the design, conduct, recording, and reporting of investigations involving medical devices. Its key components are critical for ensuring the integrity and success of clinical trials:
- Research Design: A robust research design is fundamental, addressing the specific objectives of the investigation while prioritizing participant safety. A well-structured design significantly enhances the reliability of outcomes and facilitates regulatory approval. bioaccess® specializes in managing various research types, including Early-Feasibility Trials (EFS), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-Market Clinical Follow-Up Trials (PMCF), ensuring that each trial is tailored to meet the highest standards of quality and compliance. With over 20 years of experience in Medtech, bioaccess® brings a wealth of knowledge to each project, enhancing the likelihood of successful outcomes.
- Informed Consent: The standard mandates comprehensive informed consent processes, ensuring participants are thoroughly educated about the study's purpose, procedures, potential risks, and benefits prior to participation. This transparency is vital for ethical compliance and fosters trust between researchers and participants. bioaccess® emphasizes informed consent as a cornerstone of its clinical study management services, enhancing participant engagement and retention.
- Information Integrity: Ensuring the precision, completeness, and verifiability of information gathered during studies is a cornerstone of ISO 14155:2020. This requirement is crucial for maintaining the reliability of research results, ultimately influencing the acceptance of medical devices in the market. With over 20 years of experience in Medtech, bioaccess® applies stringent data management practices to maintain data integrity throughout the study process.
- Ethical Considerations: Adherence to ethical standards is non-negotiable. The formation of an independent ethics committee to supervise the study is a critical element, ensuring that all ethical considerations are addressed throughout the research. bioaccess® partners with local health authorities, including INVIMA, to ensure adherence to ethical standards and regulatory obligations, positioning Barranquilla as a premier destination for research studies in Latin America.
In 2025, compliance with ISO 14155:2020 has become increasingly significant, as best practices for medical device trials demonstrate that adherence to these guidelines enhances the quality of research investigations. Organizations implementing these standards report marked improvements in participant retention rates, attributed to the emphasis on informed consent and ethical practices. Notably, over 200,000 medical device professionals are now part of a community that recognizes the importance of these standards.
Examples show that businesses utilizing ISO 14155:2020 not only optimize their testing procedures but also achieve quicker market access for their medical devices, emphasizing best practices for medical device trials and the standard's influence on overall trial success. One such case study, titled "Application of ISO 14155 in Post-Market Activities," illustrates the practical application of the standard across different stages of the product lifecycle, providing clear guidance on its implementation.
Furthermore, bioaccess® has played a crucial role in assisting Medtech startups, demonstrating how customized research services can promote the development of medical devices. As Jon Ingi Bergsteinsson, co-founder of Greenlight Guru Clinical, noted, "He paved the way for the platform’s quality standards, data security, and compliance," emphasizing the critical nature of these elements in trials.
Additionally, the recent guidance from MDCG 2024-3 highlights the need for justification based on the state of the art of medical practice, reinforcing the relevance of ISO 14155:2020 in today's research landscape.

Designing Effective Clinical Investigations: Best Practices and Essential Traits
Designing effective clinical investigations necessitates meticulous planning and attention to several critical factors.
- Clear Objectives: Establishing specific, measurable objectives is paramount for guiding both the study design and subsequent analysis. A well-defined objective not only clarifies the purpose of the study but also aligns the research with regulatory expectations, ultimately enhancing the likelihood of success. This emphasis on clear goals is a foundation of bioaccess®'s strategy, ensuring that Medtech startups can manage the intricacies of research processes effectively.
- Robust Methodology: Choosing a suitable research design—such as randomized controlled experiments or cohort analyses—is essential. The chosen methodology must align with the research objectives and comply with regulatory requirements, ensuring that the investigation is both scientifically sound and ethically conducted. Bioaccess® specializes in extensive research management services, including feasibility studies and compliance evaluations, to support this process.
- Sample Size Calculation: Performing a power analysis is crucial to determine the necessary sample size for achieving statistically significant results. This step is essential, as inadequate sample sizes can lead to inconclusive findings, undermining the study's validity and potential impact on clinical practice.
- Endpoint Selection: Clearly defining primary and secondary endpoints that are clinically relevant and reliably measurable is essential. This clarity not only facilitates data collection and analysis but also ensures that the outcomes are meaningful to stakeholders, including regulatory bodies and healthcare providers.
- Pilot Tests: Carrying out pilot tests can be crucial for assessing the practicality of the design. These preliminary investigations allow researchers to identify potential challenges and make necessary adjustments before launching the full-scale trial, thereby increasing the chances of success. Bioaccess®'s expertise in Early-Feasibility and First-In-Human studies exemplifies this proactive approach.
In 2025, the focus on commercial outcomes in drug development is expected to intensify as clinical end-markets become increasingly crowded. Companies are already assessing site experiences by tracking entry efficiency, which has revealed that a significant portion of information is entered on the same day as the visit date. This efficiency not only streamlines processes but also enhances patient care through improved data management. Moreover, the incorporation of artificial intelligence and machine learning in research studies has the potential to shorten study timelines by up to 30% and decrease expenses by as much as 20%. These advancements highlight the significance of embracing best practices in research methodology to enhance outcomes and foster innovation in the medical device sector.
Bioaccess® utilizes such advancements to enhance their research services, assisting Medtech companies in improving site experiences and effectively navigating the crowded market. Furthermore, the partnership between bioaccess® and Caribbean Health Group seeks to establish Barranquilla as a premier location for research studies in Latin America, backed by Colombia's Minister of Health, which further highlights the strategic significance of these best practices.
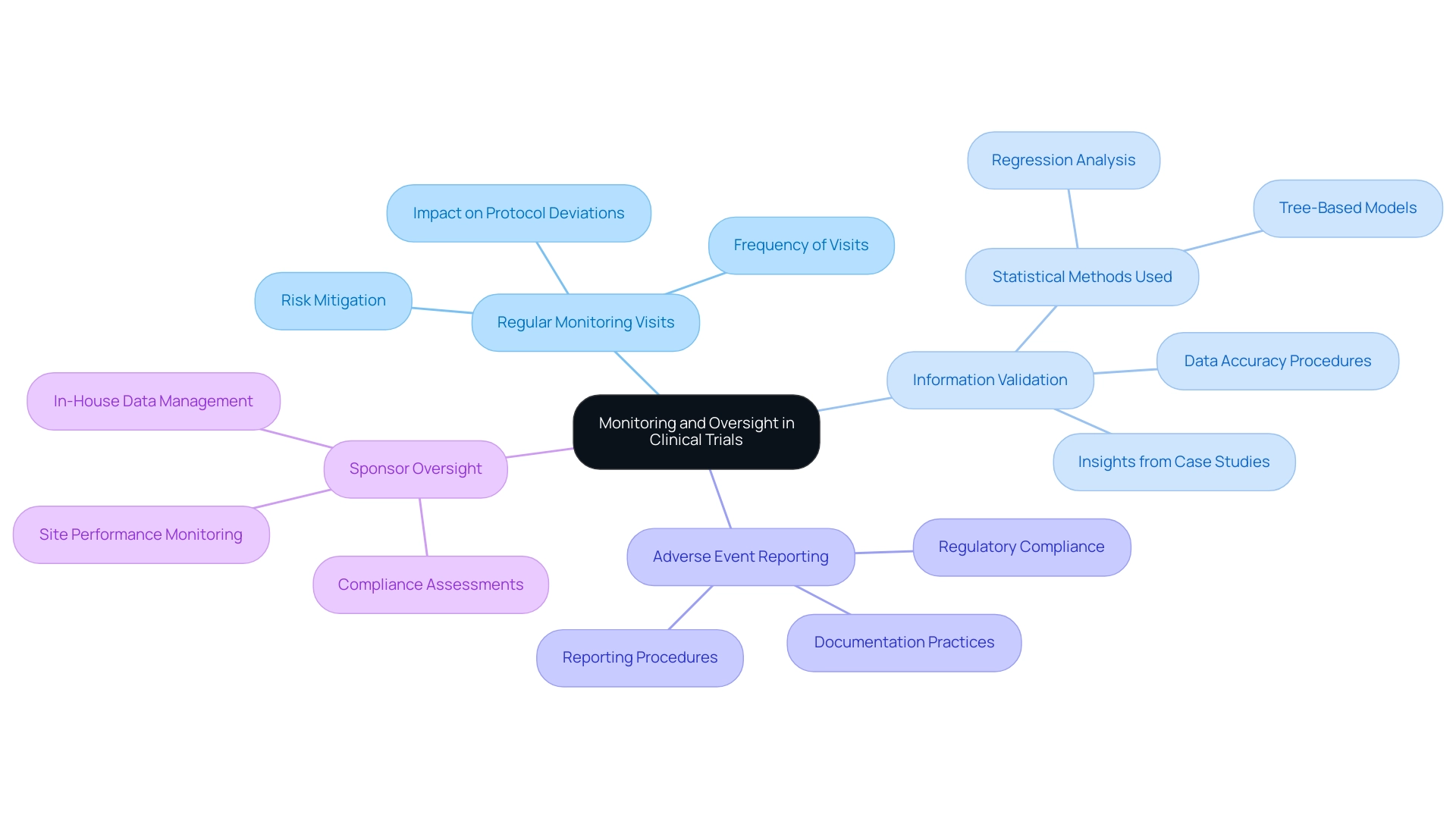
The Role of Monitoring and Oversight in Clinical Trials
Monitoring and oversight stand as essential pillars in clinical studies, ensuring strict adherence to protocols and regulatory standards. Effective strategies encompass several key practices:
- Regular Monitoring Visits: Frequent site visits are crucial for assessing compliance with study protocols, reviewing data accuracy, and ensuring participant safety. Statistics reveal that regular monitoring visits can significantly diminish the incidence of protocol deviations, thereby enhancing the overall quality of the study. For instance, the Poisson distribution suggests that a minimum of three visits is recommended to effectively manage and mitigate risks associated with research sites. Bioaccess provides extensive project management services that incorporate these regular monitoring visits, ensuring comprehensive supervision of all study aspects.
- Information Validation: Establishing robust procedures to confirm the precision and thoroughness of information gathered during experiments is vital. This includes cross-checking entries and employing advanced statistical methods, such as regression analysis, to identify discrepancies. A recent case analysis demonstrated that utilizing a combination of tree-based models and Ordinary Least Squares (OLS) regression can effectively forecast clinical study duration, underscoring the significance of information integrity in optimizing timelines. This analysis revealed varying degrees of correlation between complexity and duration across different phases, offering insights into the factors influencing both duration and complexity. At bioaccess, we prioritize data verification as part of our commitment to regulatory excellence and quality assurance.
- Adverse Event Reporting: Establishing clear procedures for reporting and managing adverse events is critical for safeguarding participant safety and maintaining study integrity. In 2025, the emphasis on adverse event reporting has intensified, with regulatory authorities underscoring the necessity for prompt and clear communication regarding any incidents occurring during the study. Bioaccess ensures meticulous documentation and reporting of all adverse events in compliance with local regulations, including those mandated by INVIMA, the Colombian National Food and Drug Surveillance Institute.
- Sponsor Oversight: Active oversight by sponsors is essential to ensure compliance with regulatory standards and adherence to best practices in medical device trials. This includes regular assessments of site performance and participant safety, which are fundamental for preserving the trial's credibility. As noted by the Head of Clinical Information Engineering, "Traditionally, information management was outsourced to our CRO vendor partners." The initiative aims to bring all research in-house, enabling our internal teams to engage more directly. This approach allows us to operationalize studies internally, take control of our data, and deliver high-quality outcomes for our patients. Expert opinions emphasize that best practices for medical device trials, including effective sponsor oversight, can lead to improved outcomes and enhanced trust in the research process. At bioaccess, our skilled Clinical Study Managers, such as Oswaldo Amaya, MD, and Dr. Sergio Alvarado, are dedicated to ensuring that all studies are conducted with the utmost integrity and compliance.
By implementing best practices for medical device trials, research studies can achieve elevated standards of quality and safety, ultimately yielding more dependable outcomes and accelerating advancements in medical technology.
Leveraging Technology: Best Practices for eCRF in Medical Device Trials
Implementing electronic case report forms (eCRFs) in medical device studies is essential for streamlining data collection and significantly enhancing data quality. At bioaccess®, we recognize that adopting best practices for medical device trials can lead to improved outcomes and efficiency in research. Our comprehensive clinical trial management services encompass feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting, ensuring that your clinical trials are conducted with the utmost expertise and care.
We specialize in various types of research, including Early-Feasibility Assessments (EFS), First-In-Human Trials (FIH), Pilot Investigations, Pivotal Evaluations, and Post-Market Clinical Follow-Up Assessments (PMCF). Key strategies include:
- User-Friendly Design: Prioritize an intuitive and easy-to-navigate interface for eCRFs. An intuitive design reduces the risk of mistakes during information input, which is essential for preserving integrity and reliability. Transparency in eCRF processes can enhance trust and loyalty among stakeholders, as studies indicate that 94% of customers exhibit greater loyalty to brands that are open about their operations.
- Real-Time Information Entry: Utilize eCRFs that support real-time information entry. This capability enables prompt analysis and informed decision-making, ultimately speeding up the clinical trial process and improving responsiveness to emerging trends.
- Automated Validation Checks: Integrate automated validation checks within the eCRF framework. These checks can promptly identify and rectify entry errors, ensuring that the information collected is accurate and compliant with regulatory standards.
- Integration with Other Systems: Ensure that the eCRF is compatible with other information management systems. This integration facilitates seamless information flow, reduces redundancy, and enhances overall operational efficiency. Selecting appropriate eCRF software is crucial, featuring an eCRF library for reusability, regulatory compliance support, ease of use, and support for amendments and version control.
The influence of an intuitive design on eCRF quality cannot be exaggerated; research shows that 94% of customers display increased loyalty to brands that uphold transparency in their operations. Moreover, the advantages of real-time information entry in medical studies are underscored by recent figures, showcasing its importance in enhancing accuracy and accelerating the research process. A case study on regulatory compliance in research databases emphasizes the necessity for research databases to comply with regulatory standards set by agencies such as INVIMA, FDA, and EMA, focusing on the importance of audit trails and data traceability in ensuring data integrity.
Organizations can enhance their studies by adhering to best practices for medical device trials, ensuring compliance with regulatory requirements while promoting the development of innovative medical devices. As noted by Nelly Zental, "M.G. designed the integration between EDC and EHR and prepared the final version of the manuscript," highlighting the importance of expert involvement in the design and implementation of eCRFs.
Challenges and Opportunities in Latin American Medical Device Trials
Conducting medical device studies in Latin America presents a complex landscape characterized by both challenges and opportunities.
- Regulatory Variability: The regulatory environment in Latin America is fragmented, with each country maintaining its own set of rules and guidelines. This variability can result in delays in the approval process for research studies, as companies must navigate differing requirements and standards. For instance, the approval timelines can vary significantly, impacting the overall speed of bringing innovative medical devices to market. Bioaccess® leverages its expertise in navigating these complexities, ensuring compliance and facilitating smoother trial setups, drawing on over 20 years of experience in the Medtech industry.
- Cultural Considerations: Effective participant recruitment and retention hinge on a deep understanding of cultural nuances and local practices. In countries like Peru, Chile, and Colombia, regional dialects and cultural influences can affect communication strategies. As Larissa Aviles-Santa, Director of the Division of Health Services Research, emphasizes, understanding local idioms is crucial to avoid misunderstandings in health research. Customizing recruitment strategies to connect with local communities is vital for improving participant involvement and guaranteeing the success of trials.
- Cost-Effectiveness: Latin America stands out as a cost-effective option for conducting medical studies. The operational costs in this region are generally lower than those in North America and Europe, making it an attractive option for Medtech companies looking to optimize their budgets while maintaining high-quality research standards. This economic benefit enables broader research and quicker patient recruitment, further backed by bioaccess's all-encompassing study management services, which consist of feasibility assessments, site selection, and project oversight.
- Diverse Patient Populations: The region's rich diversity in patient demographics offers a unique opportunity to gather insights into the safety and efficacy of medical devices across various populations. This diversity can enhance the generalizability of study results, providing valuable data that can inform future product development and regulatory submissions. Bioaccess's partnership with Caribbean Health Group seeks to establish Barranquilla as a prominent location for medical studies, further boosting the region's attractiveness for Medtech research.
In 2022, the research landscape experienced around 106 early phase-I interventional studies and a total of 2,478 phase-I interventional studies, underscoring the strong activity in this sector. The oncology segment, in particular, has been a focal point, driven by the increasing prevalence of cancer and the demand for innovative treatments. The findings indicate that economic, population, and regulatory factors significantly affect the geographical distribution of research studies.
As the need for research trials continues to grow, understanding the specific challenges and opportunities in Latin America will be essential for applying the best practices for medical device trials and ensuring successful trial execution and product advancement. Bioaccess® specializes in various study types, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring a comprehensive approach to clinical research.
Conclusion
In the intricate realm of medical device trials, understanding and implementing key principles and best practices is crucial for ensuring the safety and efficacy of new technologies. Adherence to Good Clinical Practice (GCP) and ISO 14155:2020 guidelines not only safeguards participant rights but also enhances data integrity and regulatory compliance. As highlighted throughout the article, effective risk management, robust data management, and a well-structured study design are fundamental elements that contribute to successful trial outcomes.
Navigating the unique challenges and opportunities within the Latin American context further emphasizes the significance of cultural considerations and regulatory variability. The region’s diverse patient populations provide a rich landscape for gathering valuable insights, while its cost-effectiveness presents an attractive alternative for conducting clinical trials. Organizations like bioaccess continue to leverage their expertise in these areas, creating substantial potential for innovation and advancement in medical technology.
Ultimately, the integration of these foundational elements fosters excellence in clinical research and cultivates trust among stakeholders. By prioritizing these best practices and embracing the evolving landscape of clinical trials, organizations can enhance their capabilities, drive innovation, and contribute to improved patient outcomes and local economies. As the medical device industry continues to evolve, a commitment to excellence in trial design and execution will be paramount for achieving sustainable success.
Frequently Asked Questions
What are the essential principles governing medical device evaluations?
The essential principles include Good Clinical Practice (GCP), Regulatory Compliance, Risk Management, and Information Management, which ensure the safety and efficacy of medical devices being tested.
What is Good Clinical Practice (GCP) and why is it important?
GCP refers to guidelines that maintain the integrity of clinical trials, ensuring informed consent, participant rights protection, and information integrity. Adherence to GCP is critical for data management and the overall success of clinical research.
How does regulatory compliance affect clinical studies?
Regulatory compliance ensures a thorough understanding of local and international regulations, which influences the approval process and success of medical devices in the market. Compliance with bodies like the FDA, EMA, and INVIMA is essential.
What role does risk management play in medical device studies?
Risk management involves identifying and mitigating risks to ensure participant safety and study integrity. Comprehensive risk assessments and strategies to minimize potential adverse effects are vital for successful studies.
Why is information management crucial in clinical trials?
Efficient information management guarantees the reliability of test outcomes. Employing validated data collection tools and maintaining accurate records are essential for achieving trustworthy results in clinical research.
What is ISO 14155:2020, and what does it encompass?
ISO 14155:2020 outlines essential requirements and best practices for medical device trials, covering the design, conduct, recording, and reporting of investigations to ensure the integrity and success of clinical trials.
How does research design impact clinical trials?
A robust research design addresses specific study objectives while prioritizing participant safety, enhancing the reliability of outcomes and facilitating regulatory approval.
What is the significance of informed consent in clinical studies?
Informed consent ensures participants are fully educated about the study's purpose, procedures, risks, and benefits, fostering trust and ethical compliance in research.
How does information integrity affect research results?
Ensuring the precision, completeness, and verifiability of information is crucial for maintaining the reliability of research results, influencing the acceptance of medical devices in the market.
What ethical considerations are involved in medical device trials?
Adherence to ethical standards is essential, including the formation of an independent ethics committee to oversee studies and ensure all ethical considerations are addressed.
How has ISO 14155:2020 impacted participant retention rates?
Organizations implementing ISO 14155:2020 report improvements in participant retention rates due to its emphasis on informed consent and ethical practices.
What advantages do businesses gain from implementing ISO 14155:2020?
Businesses that utilize ISO 14155:2020 can optimize testing procedures and achieve quicker market access for their medical devices, enhancing overall trial success.
How does bioaccess® contribute to medical device research?
Bioaccess® specializes in managing various types of research trials, ensuring compliance with high standards and enhancing the likelihood of successful outcomes through tailored services.
List of Sources
- Fundamentals of Medical Device Trials: Key Principles and Practices
- 2025 Clinical Data Trend Report | Veeva (https://veeva.com/2025-clinical-data-trend-report)
- SCOPE Summit 2025: Outlook on Clinical Data (https://appliedclinicaltrialsonline.com/view/scope-summit-2025-outlook-clinical-data)
- Good Clinical Practice Guidance and Pragmatic Clinical Trials: Balancing the Best of Both Worlds - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC4777975)
- Large simple randomized controlled trials—from drugs to medical devices: lessons from recent experience - Trials (https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-025-08724-x)
- Navigating Regulatory Frameworks: Understanding ISO 14155:2020
- New Guidance on Clinical Investigation Plans for Medical Devices (https://emergobyul.com/news/new-guidance-clinical-investigation-plans-medical-devices)
- How to Set Up Clinical Studies to Comply with US FDA Regulations (https://greenlight.guru/blog/how-to-set-up-clinical-studies-to-comply-with-us-fda-regulations)
- ISO 14155:2020 (https://iso.org/standard/71690.html)
- Ultimate Guide to ISO 14155:2020 for Medical Devices (https://greenlight.guru/blog/iso-14155)
- Designing Effective Clinical Investigations: Best Practices and Essential Traits
- Navigating the Future of Clinical Trials: Expert Insights for 2025 | Novotech CRO (https://novotech-cro.com/articles/navigating-future-clinical-trials-expert-insights-2025)
- 2025 Clinical Data Trend Report | Veeva (https://veeva.com/2025-clinical-data-trend-report)
- Clinical Trial Trends & Insights for 2025 | WCG (https://wcgclinical.com/insights/clinical-trial-trends-insights-2025)
- The Role of Monitoring and Oversight in Clinical Trials
- Clinical trials are becoming more complex: a machine learning analysis of data from over 16,000 trials - Scientific Reports (https://nature.com/articles/s41598-024-53211-z)
- Determining the extent and frequency of on-site monitoring: a bayesian risk-based approach - BMC Medical Research Methodology (https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-024-02261-y)
- 2025 Clinical Data Trend Report | Veeva (https://veeva.com/2025-clinical-data-trend-report)
- Leveraging Technology: Best Practices for eCRF in Medical Device Trials
- The 10 Top Customer Experience Statistics for 2025 + How To’s | Giva (https://givainc.com/blog/customer-experience-statistics)
- Next-generation study databases require FAIR, EHR-integrated, and scalable Electronic Data Capture for medical documentation and decision support - npj Digital Medicine (https://nature.com/articles/s41746-023-00994-6)
- Effective eCRF Design: A Comprehensive Guide - Klindat (https://klindat.com/effective-ecrf-design-a-comprehensive-guide)
- Challenges and Opportunities in Latin American Medical Device Trials
- Clinical Trials in Latin America (https://languageconnections.com/clinical-trials-in-latin-america)
- Clinical Trials Market Forecast Report 2025: A $99.25 Billion Industry by 2033, Driven by Acceptance of Decentralized Experiments, Shift Towards Personalized Medicine, Demand for Effective Treatments (https://globenewswire.com/news-release/2025/03/10/3039882/28124/en/Clinical-Trials-Market-Forecast-Report-2025-A-99-25-Billion-Industry-by-2033-Driven-by-Acceptance-of-Decentralized-Experiments-Shift-Towards-Personalized-Medicine-Demand-for-Effect.html)
- Current globalization of drug interventional clinical trials: characteristics and associated factors, 2011–2013 - Trials (https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-017-2025-1)
- Expired PAR-23-304: Interventions on Health and Healthcare Disparities on Non-Communicable and Chronic Diseases in Latin America: Improving Health Outcomes Across the Hemisphere (R01 - Clinical Trial Required) (https://grants.nih.gov/grants/guide/pa-files/PAR-23-304.html)





