


Multicenter trials play a crucial role in advancing medical research, yet coordinating them can often feel like navigating a complex maze. In Montenegro, where diverse teams and locations converge, establishing effective communication channels and standardized protocols is not just beneficial; it’s essential for success. This article explores best practices for coordinating multicenter trials in this unique landscape, emphasizing the significance of local expertise and robust monitoring systems.
How can researchers ensure their trials not only meet regulatory standards but also foster collaboration and efficiency across various sites?
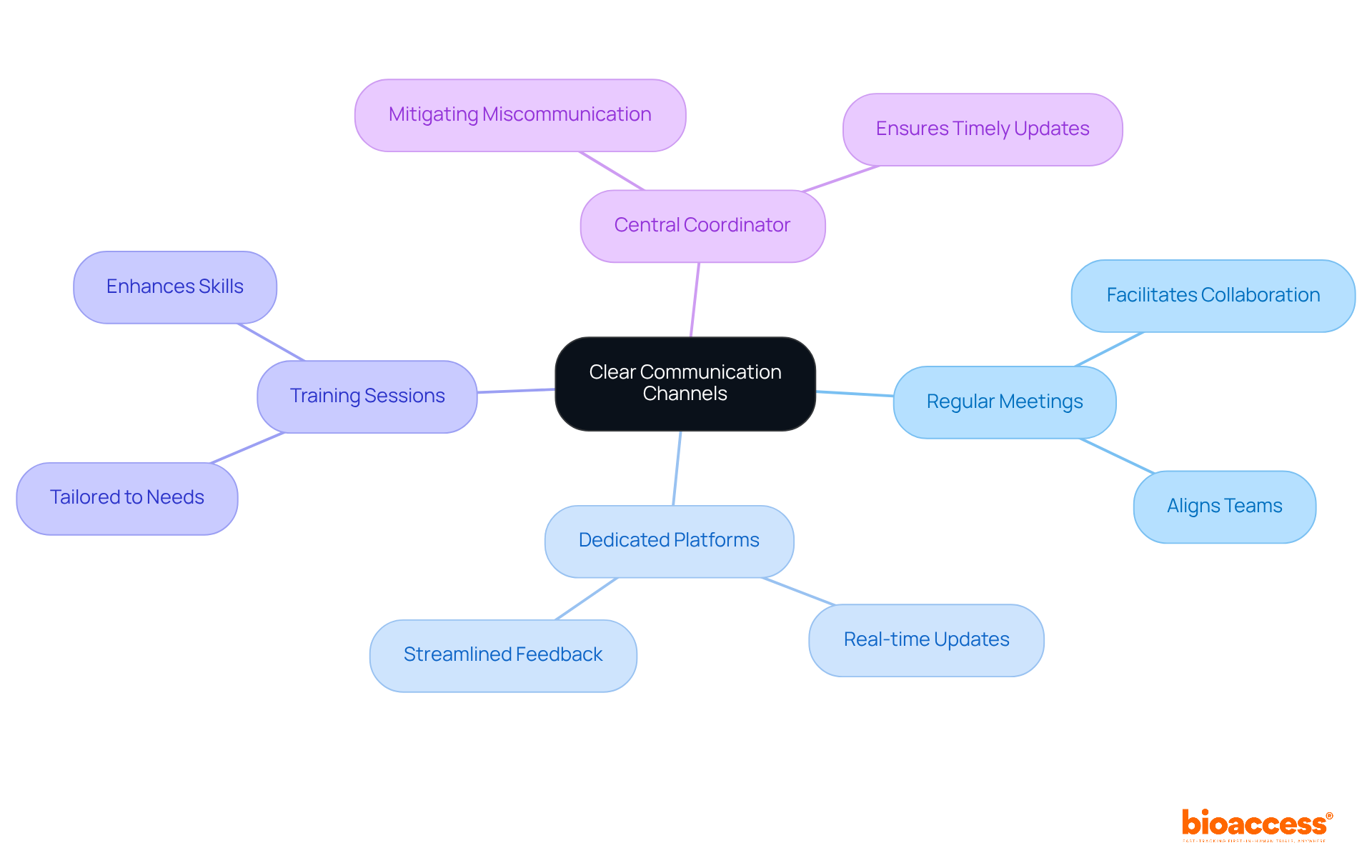
Creating transparent communication pathways among all engaged locations is crucial for the success of multicenter trial coordination in Montenegro. Regular meetings, dedicated communication platforms, and thorough training sessions tailored to each location's needs can achieve this goal. By leveraging project management software, teams can streamline communication, enabling real-time updates and feedback - essential for maintaining alignment across diverse teams.
Designating a central coordinator is vital; this individual facilitates communication between locations, ensuring that all parties are promptly informed of any modifications or updates to the protocol. Such proactive measures not only mitigate the risk of miscommunication but also foster a collaborative atmosphere, ultimately enhancing the efficiency and effectiveness of the process. Research directors emphasize that effective communication strategies are essential for multicenter trial coordination in Montenegro, as they directly influence recruitment, retention, and overall study results.
For instance, bioaccess's accelerated patient recruitment services and pre-qualified networks, with over 50 sites activated in less than eight weeks, can significantly bolster recruitment efforts. A study highlighted that investing in patient support services leads to improved research outcomes, underscoring the importance of clear communication from the outset to ensure patients feel valued and supported throughout the study. Furthermore, the economic costs of miscommunication in clinical studies can be substantial, making it imperative to implement robust communication strategies to avoid common pitfalls.
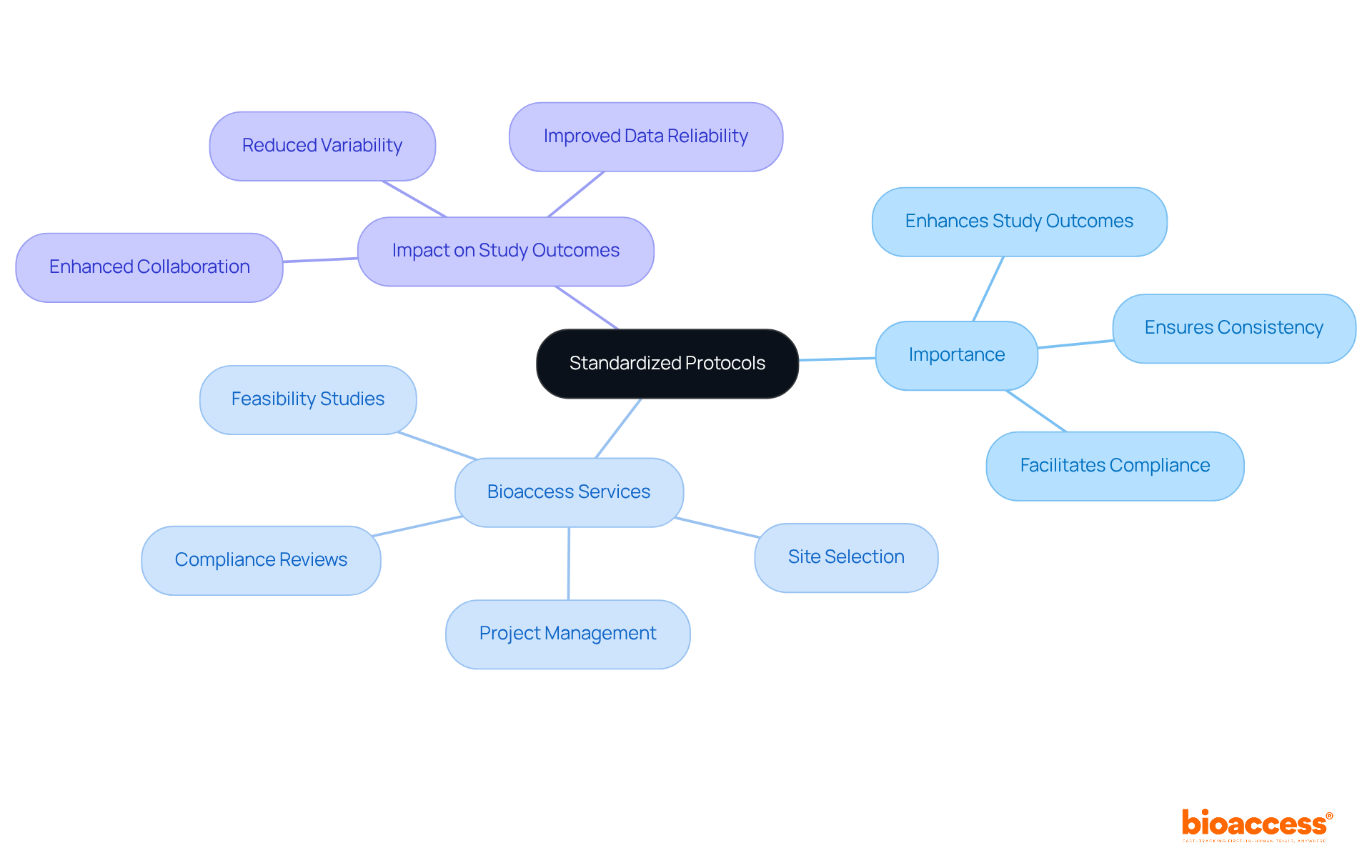
Standardized protocols are essential for the success of multicenter trial coordination in Montenegro, ensuring that all participating locations adhere to the same procedures and guidelines. A well-organized trial protocol should clearly outline the research design, eligibility criteria, and data collection methods. To achieve this, thorough training for each location is crucial, guaranteeing consistent application of these protocols.
Bioaccess plays a pivotal role in this landscape, offering extensive services such as:
These services are vital in establishing standardized protocols that enhance the reliability of test results and ensure adherence to regulatory requirements. Regular audits and feedback mechanisms can pinpoint areas for improvement, fostering a culture of compliance and excellence.
As noted by medical researchers, the application of standardized protocols significantly enhances study outcomes. This underscores the importance of protocol adherence in achieving consistent and reproducible results across diverse research environments. By collaborating with Bioaccess, clinical research teams can navigate the complexities of multicenter trial coordination in Montenegro, ensuring that their protocols not only meet but exceed industry standards.
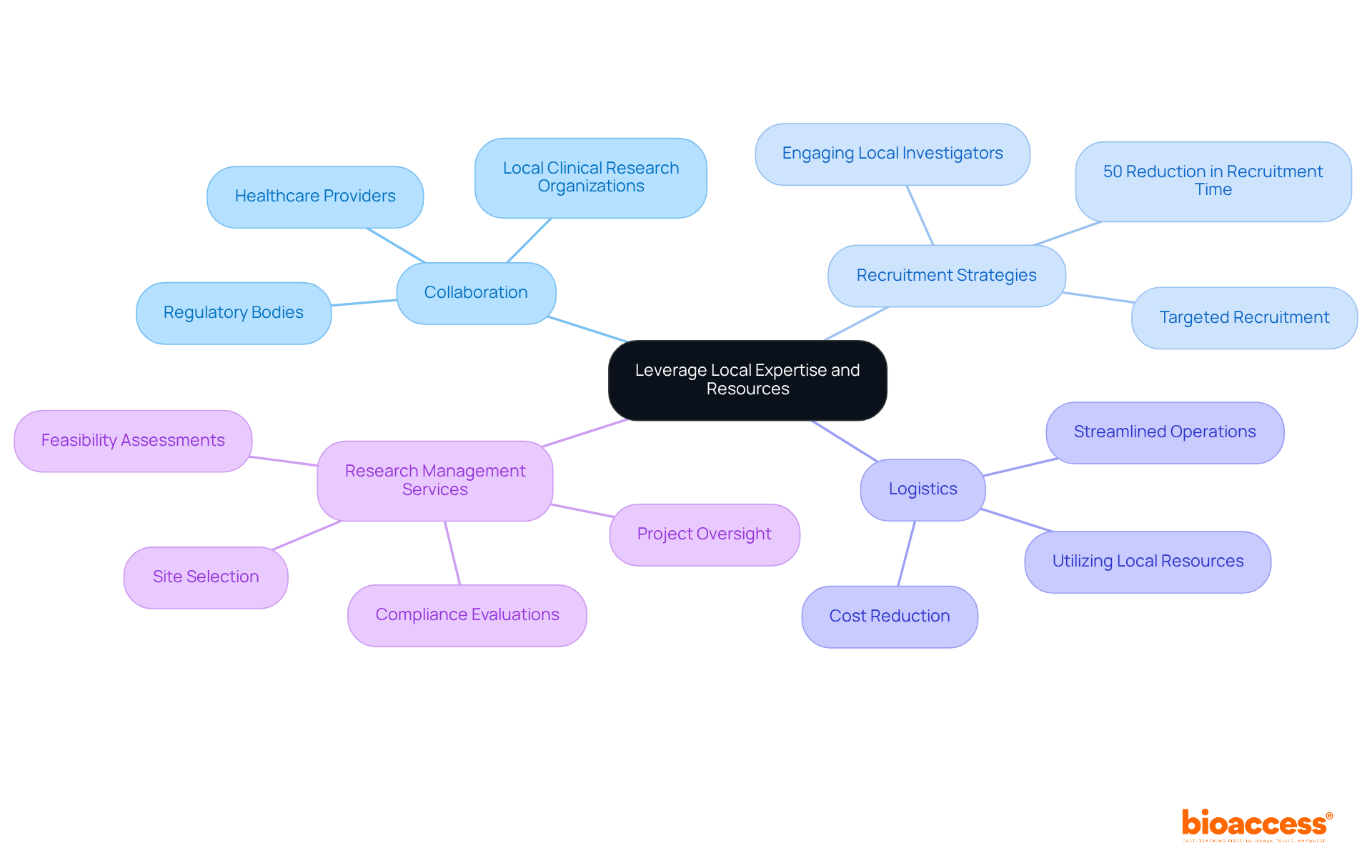
Maximizing the success of multicenter trial coordination in Montenegro is crucial for advancing clinical research. Effectively leveraging local expertise and resources is key to achieving this goal. Collaborating with local clinical research organizations, healthcare providers, and regulatory bodies provides invaluable insights into the regional healthcare landscape. Engaging local investigators who understand the patient population enhances recruitment efforts and improves participant retention rates.
For instance, targeted recruitment strategies have been shown to significantly increase enrollment, with local networks contributing to a remarkable 50% reduction in recruitment time in some cases. Furthermore, utilizing local resources for logistics and patient management streamlines operations and reduces costs, making studies more efficient. By tapping into local expertise, researchers can navigate regulatory requirements more easily, ensuring compliance and improving overall efficiency. This collaborative approach not only builds trust within the community but also enhances multicenter trial coordination in Montenegro, aligning research objectives with local health needs and ultimately leading to more successful outcomes.
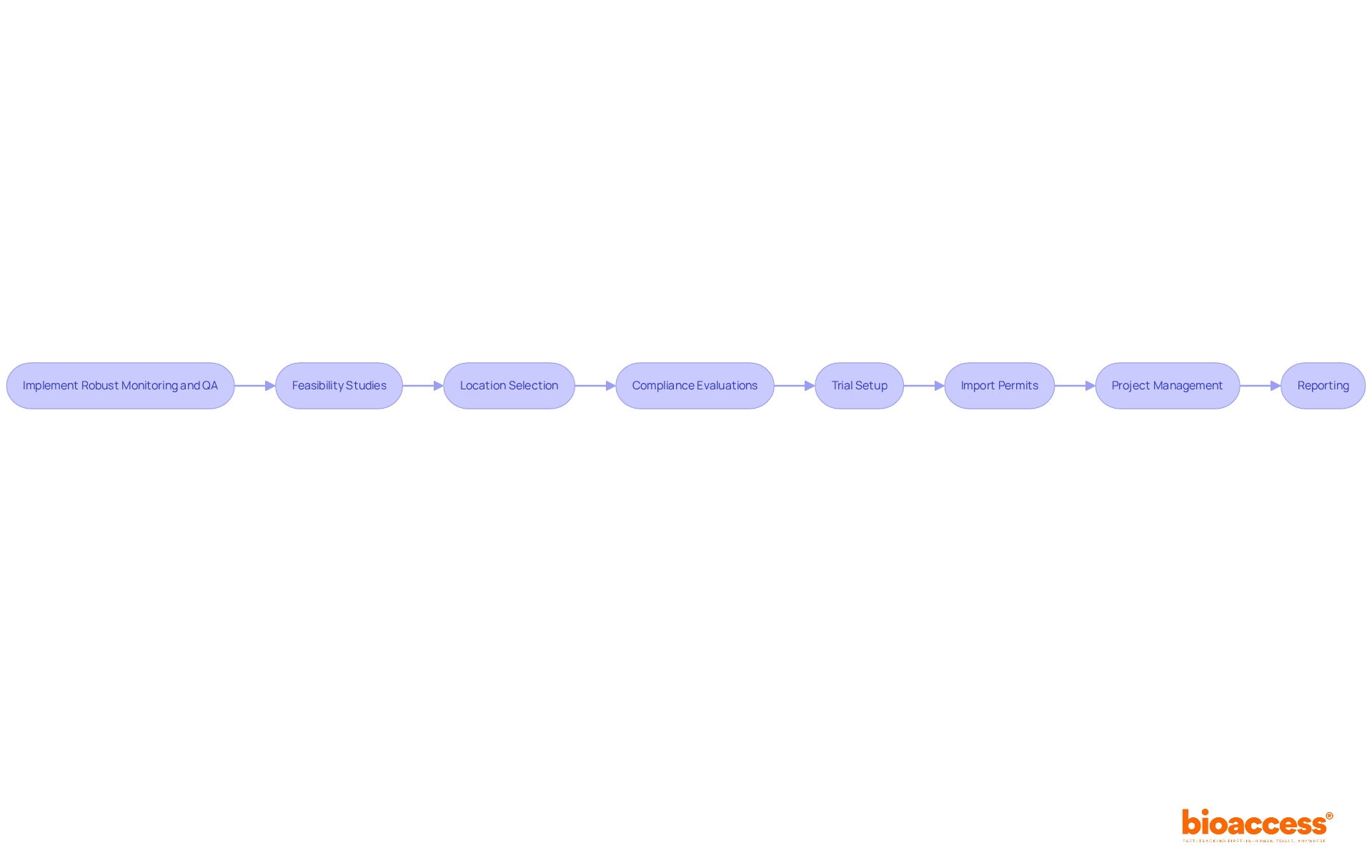
Moreover, extensive medical research management services - such as feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting - are essential for achieving these successful outcomes. Ultimately, these efforts enhance the influence of Medtech research on local economies, fostering job creation, economic growth, and healthcare improvement while promoting international collaboration and innovation in the Medtech sector.
To ensure the integrity of multicenter trial coordination in Montenegro, it is essential to implement robust monitoring and quality assurance (QA) processes. Bioaccess offers extensive clinical trial management services, including:
A thorough monitoring strategy that details the frequency and methods of location visits, data verification, and compliance checks is crucial.
Utilizing technology, such as electronic data capture systems, facilitates real-time monitoring and enhances data accuracy. Regular training sessions for site staff on QA practices further reinforce the importance of compliance. By prioritizing monitoring and QA, researchers involved in multicenter trial coordination in Montenegro can promptly identify and address any issues, ensuring the trial's success and the safety of participants.
In the ever-evolving Medtech landscape, collaboration is key. Bioaccess stands ready to address the challenges faced in clinical research, ensuring that trials are conducted with the utmost integrity and efficiency. The next steps involve engaging with experts who can guide you through the complexities of clinical trials, ensuring that your research not only meets regulatory standards but also achieves its objectives.
Effective multicenter trial coordination in Montenegro relies on several best practices that significantly enhance communication, standardization, local engagement, and quality assurance. By establishing clear communication channels, developing standardized protocols, leveraging local expertise, and implementing robust monitoring systems, researchers can markedly improve clinical trial outcomes. These strategies not only streamline operations but also foster collaboration and trust among all stakeholders involved.
Key insights from this discussion underscore the importance of transparent communication pathways, which mitigate risks associated with miscommunication and enhance participant engagement. The role of standardized protocols is equally crucial, ensuring consistency and adherence to regulatory requirements across various sites. Furthermore, tapping into local expertise and resources can expedite recruitment and improve overall trial efficiency, while robust monitoring and quality assurance processes safeguard the integrity of research.
In light of these findings, it is essential for researchers and organizations involved in multicenter trials in Montenegro to adopt these best practices. By prioritizing effective communication, standardization, local collaboration, and diligent monitoring, the clinical research community can achieve successful trial outcomes and contribute to the advancement of healthcare in the region. Embracing these strategies will ultimately lead to more reliable research, benefiting both the scientific community and the patients they serve.
Why are clear communication channels important in multicenter trial coordination in Montenegro?
Clear communication channels are crucial for successful multicenter trial coordination as they help maintain alignment across diverse teams, facilitate real-time updates and feedback, and ultimately enhance the efficiency and effectiveness of the process.
What methods can be used to establish effective communication in multicenter trials?
Effective communication can be established through regular meetings, dedicated communication platforms, thorough training sessions tailored to each location's needs, and the use of project management software.
What role does a central coordinator play in multicenter trial communication?
A central coordinator facilitates communication between locations, ensuring that all parties are promptly informed of any modifications or updates to the protocol, which helps mitigate the risk of miscommunication.
How does effective communication impact recruitment and retention in clinical studies?
Effective communication strategies directly influence recruitment, retention, and overall study results, making it essential to keep participants informed and supported throughout the study.
What are the benefits of investing in patient support services during clinical trials?
Investing in patient support services leads to improved research outcomes and can significantly bolster recruitment efforts, as it ensures that patients feel valued and supported throughout the study.
What are the economic implications of miscommunication in clinical studies?
The economic costs of miscommunication in clinical studies can be substantial, highlighting the importance of implementing robust communication strategies to avoid common pitfalls.