


Bioburden denotes the number of viable microorganisms present on a surface or within a sample prior to sterilization, playing a pivotal role in ensuring the safety and efficacy of medical devices and pharmaceuticals in the realm of clinical research.
This article underscores the necessity of effective microbial load management to avert costly product recalls and protect patient health.
The reality of numerous recalls stemming from contamination issues, coupled with stringent regulatory requirements mandating microbial load assessments, highlights the critical importance of this topic.
Understanding bioburden is essential for ensuring the safety and efficacy of medical devices and pharmaceuticals, as it quantifies the viable microorganisms present before sterilization. This article delves into the critical role of bioburden in clinical research, emphasizing that effective microbial load management can prevent costly product recalls and safeguard patient health. Given the increasing regulatory scrutiny and the complexities of modern healthcare products, researchers must consider:
The definition of bioburden is the number of viable microorganisms present on a surface or within a sample prior to sterilization. This concept is crucial in clinical research, especially concerning devices and pharmaceuticals, as it significantly impacts product safety and effectiveness. Efficient microbial load management is essential for ensuring that medical devices are devoid of harmful microbial presence, thereby safeguarding patient health and adhering to regulatory standards. Routine microbial load assessment quantifies contamination, directing the necessary sterilization procedures to guarantee product safety.
For instance, in 2019, the FDA withdrew more than 80 drugs due to impurity concerns, underscoring the economic and health risks associated with inadequate microbial load analysis. Additionally, approximately 15,710 medication recalls occurred from 2012 to 2023 because of impurity and other issues, highlighting the critical need for microbial load assessment to avert such incidents. A notable case involved a pharmaceutical firm that effectively identified a contamination issue during standard microbial assessments, allowing them to implement corrective measures and avert a costly recall. Routine microbial load assessments could have saved companies millions by preventing recalls, showcasing the financial implications of effective microbial load management.
As the global microbial load assessment market is anticipated to reach USD 3.22 billion by 2030, driven by increasing investments in research and development, the emphasis on microbial load in device safety will continue to grow. Furthermore, incorporating insights on outsourcing contamination assessment can lead to cost reductions by sidestepping the substantial expenses associated with establishing an internal laboratory, positioning this approach as a strategic consideration for clinical research leaders.
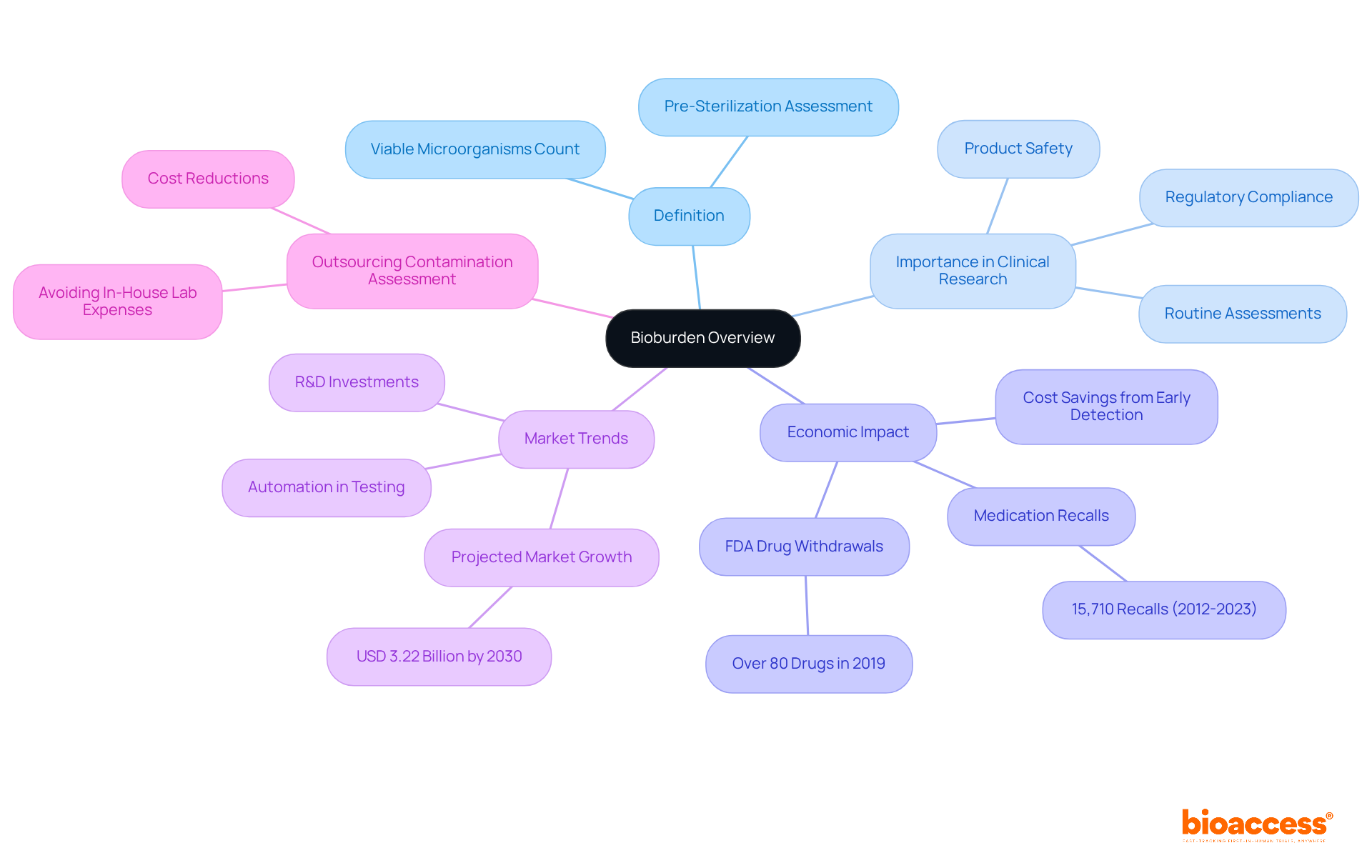
The bioburden definition plays a crucial role in the evaluation process of healthcare devices and pharmaceuticals, acting as an important quality assurance measure to ensure that products are free of microbial impurities before human trials or market introduction. The consequences of insufficient management of microbial load are significant; increased levels of microbial presence can lead to expensive product recalls, regulatory fines, and, most importantly, threatened patient safety. Regulatory agencies, such as the FDA and EMA, require microbial load assessment as described by the bioburden definition under Good Manufacturing Practices (GMP) and ISO 11737 as a component of the approval process for medical devices and pharmaceuticals, highlighting its significance in clinical research protocols.
For instance, from 2012 to 2023, more than 15,710 medication recalls occurred due to impurity problems, underscoring the urgent need for thorough microbial load testing to protect consumer health. Furthermore, 21% of consumers forsake a producer following a recall, illustrating the reputational dangers linked to poor contamination control. By efficiently comprehending and managing microbial load through techniques like the Membrane Filtration and Pour Plate methods, researchers can greatly diminish the hazards linked to microbial presence. This proactive approach not only enhances compliance with regulatory standards but also fosters greater consumer confidence in the safety and reliability of healthcare innovations.
In summary, the effective management of microbial load is essential, as highlighted in the bioburden definition, and it is not just a regulatory requirement; it is a critical factor in ensuring patient safety and maintaining the integrity of clinical trials. Collaboration among stakeholders in the Medtech landscape is essential to address these key challenges, paving the way for advancements that prioritize both safety and efficacy in healthcare products.

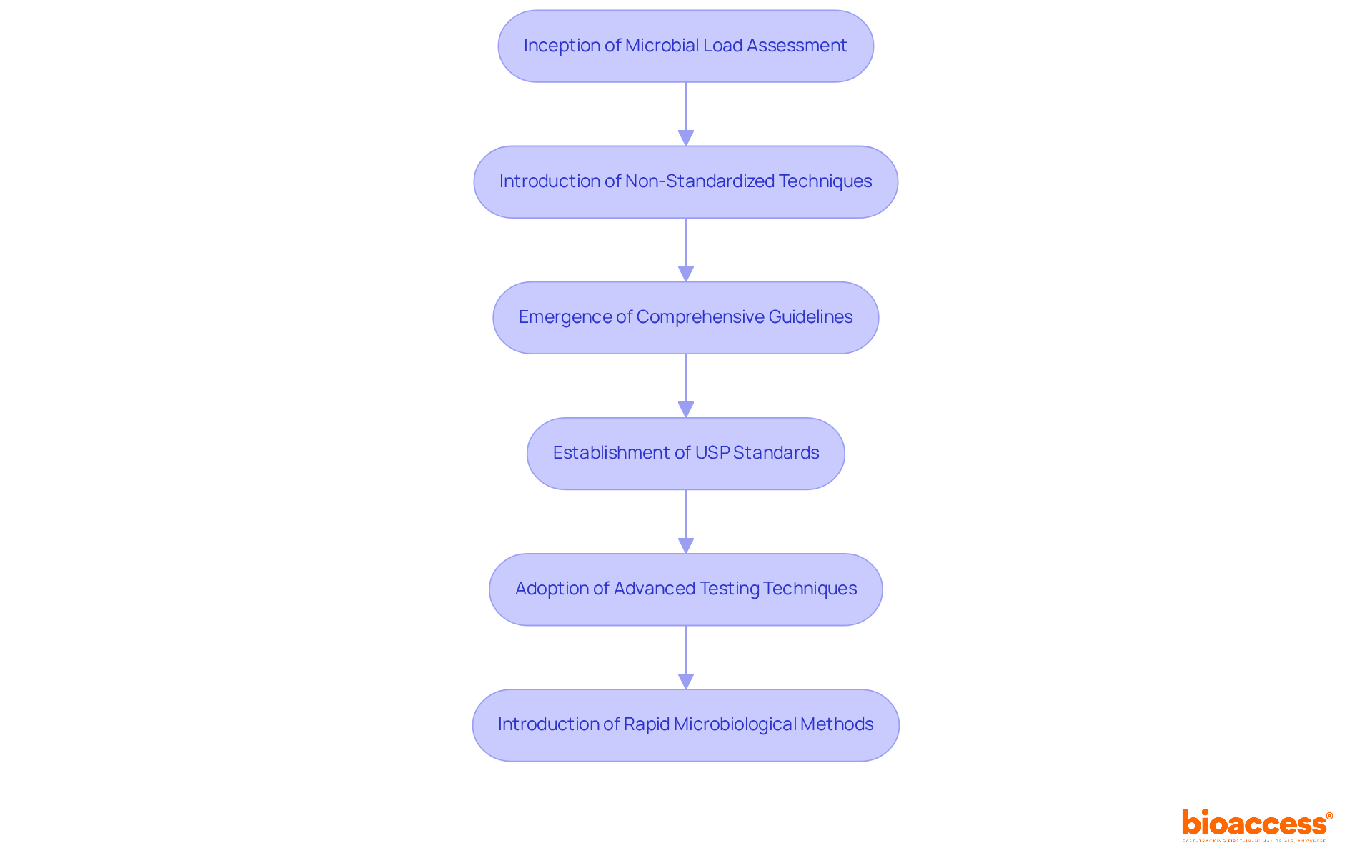
The development of microbial load assessment has undergone significant improvements since its inception, when microbial contamination was evaluated using simple, non-standardized techniques. As the critical need for cleanliness in healthcare products gained recognition, regulatory bodies began to establish comprehensive guidelines and standards for microbial load assessment. A pivotal advancement occurred with the introduction of the United States Pharmacopeia (USP) standards in the late 20th century, which formalized the bioburden definition and evaluation protocols, providing a consistent framework for reliable and reproducible assessments.
In recent years, the increasing complexity of healthcare products has necessitated further evolution in testing methodologies. Advanced techniques, such as membrane filtration and automated microbial detection systems, have emerged, reflecting the industry's response to stringent regulatory requirements and the growing sophistication of biopharmaceuticals and medical devices. For instance, a study highlighted that peaks in microbial load data—defined as values three or more times the average—can lead to an increase in the necessary sterilization dose by up to 10%, underscoring the importance of precise microbial assessments.
Moreover, the global microbial load assessment market is projected to expand significantly, with forecasts indicating an increase from USD 1.44 billion in 2024 to USD 2.8 billion by 2033, driven by the pharmaceutical and biotechnology sectors. This growth is further bolstered by escalating regulatory scrutiny and the demand for effective quality assurance in product development. As noted by industry experts, the FDA mandates comprehensive microbial impurity evaluations during drug manufacturing, emphasizing the crucial role of the bioburden definition in ensuring product safety. Additionally, the FDA retracted over 80 medications in 2019 due to contamination issues, highlighting the severe consequences of inadequate examination.
The adoption of rapid microbiological methods (RMMs) has also revolutionized assessment processes, reducing evaluation times from 14 days to as few as four days, thereby enhancing compliance and safety in clinical research. Furthermore, Merck's establishment of its Microbiology Application and Training Lab illustrates industry initiatives aimed at improving microbial evaluation procedures, aligning with the ongoing advancements in contamination assessment methodologies. A notable case involved a U.S.-based orthopedic implant company facing a $5.4 million recall due to microbial contamination, which underscores the financial ramifications of insufficient microbial load assessment.
The bioburden definition involves quantifying the number of viable microorganisms in a sample in terms of colony-forming units (CFUs). This measurement process encompasses several critical steps:
Common techniques for microbial load testing include:
Precise evaluation of microbial load is essential, as it directly affects the sterilization methods required to guarantee the safety of healthcare products and pharmaceuticals for human use. Additionally, identifying the types of microorganisms present aids manufacturers in formulating effective contamination control strategies, thereby enhancing product quality and ensuring patient safety. The bioburden definition for healthcare devices typically indicates a level ranging from 0 to 150 CFUs, with levels exceeding this threshold classified as 'too numerous to count.' This understanding is vital for manufacturers to meet regulatory compliance and maintain high standards in medical device production.
Furthermore, maintaining low CFU counts is essential for easier sterilization, as higher levels can complicate the sterilization process and release harmful endotoxins from gram-negative bacteria, posing serious health risks.

Understanding the concept of bioburden is essential for ensuring the safety and efficacy of healthcare products in clinical research. This definition encapsulates the critical need for rigorous microbial load assessments to mitigate risks associated with contamination, which can lead to severe health consequences and economic losses. The emphasis on bioburden highlights its integral role in maintaining quality assurance and regulatory compliance, ultimately safeguarding patient health.
Throughout the article, the significance of bioburden is underscored by various examples, including the alarming statistics of drug recalls and the financial repercussions linked to inadequate microbial management. The evolution of testing methodologies, such as membrane filtration and rapid microbiological methods, reflects the industry's commitment to enhancing safety standards. Furthermore, the collaboration among stakeholders in the Medtech landscape is vital to address these challenges and ensure that healthcare innovations meet the highest safety benchmarks.
In conclusion, the importance of bioburden in clinical research cannot be overstated. As the landscape of healthcare products continues to evolve, so must the strategies for microbial load assessment. Embracing a proactive approach to bioburden management will not only protect patient safety but also foster consumer confidence in healthcare advancements. Stakeholders are encouraged to prioritize microbial load testing as a fundamental aspect of product development, ensuring that the integrity of clinical trials and the health of individuals remain paramount.
What is bioburden?
Bioburden refers to the number of viable microorganisms present on a surface or within a sample prior to sterilization.
Why is bioburden important in clinical research?
Bioburden is crucial in clinical research because it significantly impacts the safety and effectiveness of products, particularly medical devices and pharmaceuticals.
How does microbial load management affect patient health?
Efficient microbial load management ensures that medical devices are free from harmful microorganisms, thereby safeguarding patient health and adhering to regulatory standards.
What is the purpose of routine microbial load assessment?
Routine microbial load assessment quantifies contamination and directs necessary sterilization procedures to guarantee product safety.
Can you provide an example of the consequences of inadequate microbial load analysis?
In 2019, the FDA withdrew over 80 drugs due to impurity concerns, and from 2012 to 2023, approximately 15,710 medication recalls occurred because of impurity and other issues, highlighting the risks of inadequate microbial load analysis.
How can routine microbial load assessments save companies money?
Routine microbial load assessments can prevent costly recalls by identifying contamination issues early, potentially saving companies millions.
What is the projected market size for microbial load assessment by 2030?
The global microbial load assessment market is anticipated to reach USD 3.22 billion by 2030, driven by increasing investments in research and development.
What are the benefits of outsourcing contamination assessment?
Outsourcing contamination assessment can lead to cost reductions by avoiding the substantial expenses associated with establishing an internal laboratory, making it a strategic consideration for clinical research leaders.