

The article delineates strategies that clinical trial directors can employ to enhance the success probability of their studies. It underscores pivotal approaches, including:
Collectively, these elements significantly augment the likelihood of achieving favorable trial outcomes while streamlining the research process.
Understanding the success probability in clinical trials is essential for research directors navigating the complexities of medical experimentation. This metric not only reflects the likelihood of meeting primary endpoints but also serves as a guiding light for strategic decision-making. As the stakes rise in the competitive landscape of Medtech, Biopharma, and Radiopharma, the pressing question is: what key strategies can directors employ to enhance these probabilities and ensure their studies not only survive but thrive?
Exploring effective methodologies and best practices could very well be the difference between a groundbreaking discovery and a missed opportunity.
The success probability in clinical studies indicates the likelihood that the study will successfully meet its primary endpoints and demonstrate a favorable risk-benefit profile. This crucial metric, the success probability, is shaped by various factors, including study design, participant demographics, and the specific therapeutic area under investigation. Notably, studies that incorporate biomarkers for patient selection tend to show significantly higher success probabilities of achieving favorable outcomes compared to those that do not utilize such targeted methods.
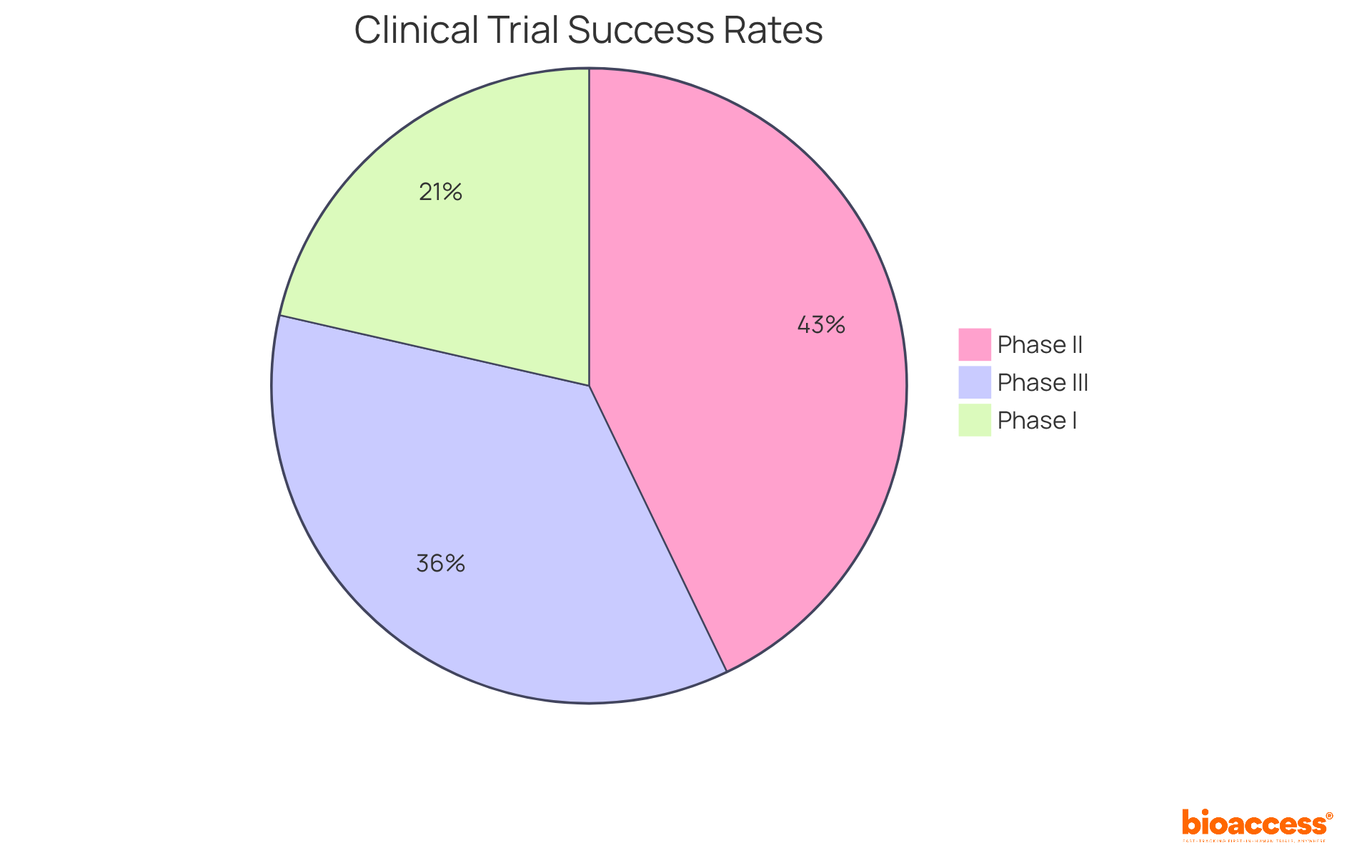
Research indicates that the typical success probability varies across different stages of medical experimentation. For instance, Phase III studies generally display a success probability of around 50%, while earlier phases, such as Phase I and II, reveal lower success probabilities of approximately 60% and 30%, respectively. By distinctly outlining the success probability of achievement, research directors can effectively communicate objectives to stakeholders and align their teams towards these goals. This strategic focus not only optimizes resource allocation but also enhances the success probability of favorable outcomes, ultimately propelling innovation within the Medtech, Biopharma, and Radiopharma sectors.
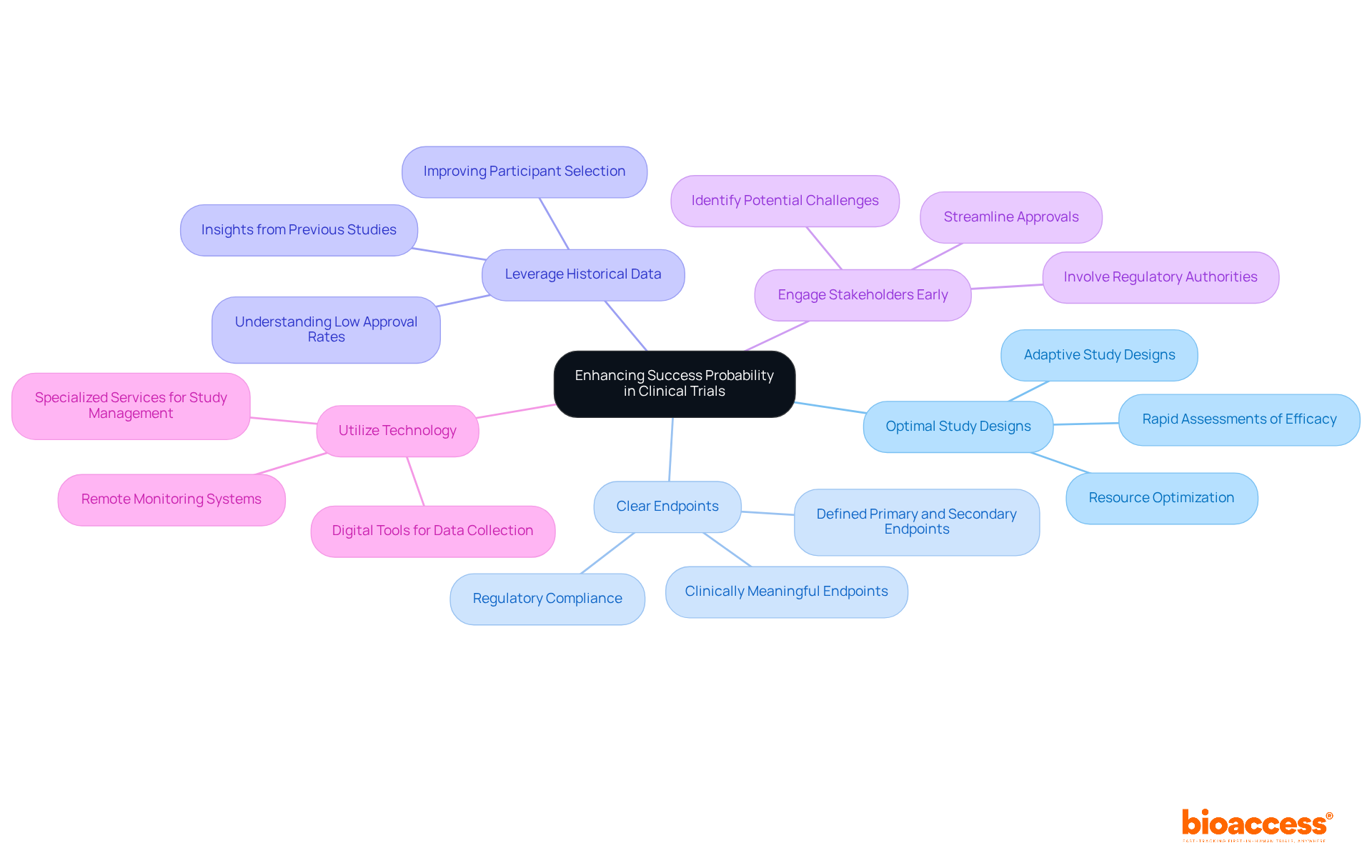
To enhance the success probability of clinical trials, directors should consider the following strategies:
Choose Optimal Study Designs: Selecting the right study design is critical. Adaptive study designs permit changes based on interim results, which can lead to more favorable outcomes. These designs facilitate rapid assessments of efficacy and futility, optimizing resource allocation and potentially increasing approval rates.
Set Clear Endpoints: Clearly defined primary and secondary endpoints are essential for accurately assessing achievement. Utilizing clinically meaningful endpoints, rather than relying solely on statistical measures, provides a comprehensive understanding of the treatment's impact. This clarity is vital for regulatory compliance and effective communication among stakeholders.
Leverage Historical Data: Using historical information from earlier studies can guide current research designs and participant selection, thereby enhancing the likelihood of success. Significantly, the success probability of endorsement from Phase 1 for all development candidates was merely 7.9 percent, highlighting the necessity of gaining insights from previous experiments to improve future results.
Engage Stakeholders Early: Involving key stakeholders, including regulatory authorities and advocacy groups for individuals, early in the study design process can help identify potential challenges and simplify approvals. Thorough planning and communication with stakeholders are crucial for ensuring that the study aligns with regulatory expectations and patient needs. With bioaccess®'s expedited regulatory approval procedure, studies can obtain approvals in merely 6-8 weeks, notably quicker than the usual 6-12 months in the US/EU.
Utilize Technology: Implementing digital tools for data collection and monitoring can enhance study efficiency and accuracy. Technologies such as electronic data capture and remote monitoring systems improve data integrity and facilitate real-time decision-making, ultimately enhancing the success probability. Moreover, bioaccess® provides specialized services in overseeing research studies, including Early-Feasibility Studies and First-In-Human Studies, ensuring a thorough approach to study management that effectively tackles patient recruitment challenges. Furthermore, bioaccess® utilizes more than 20 years of expertise in Medtech to offer customized approaches that improve study results.
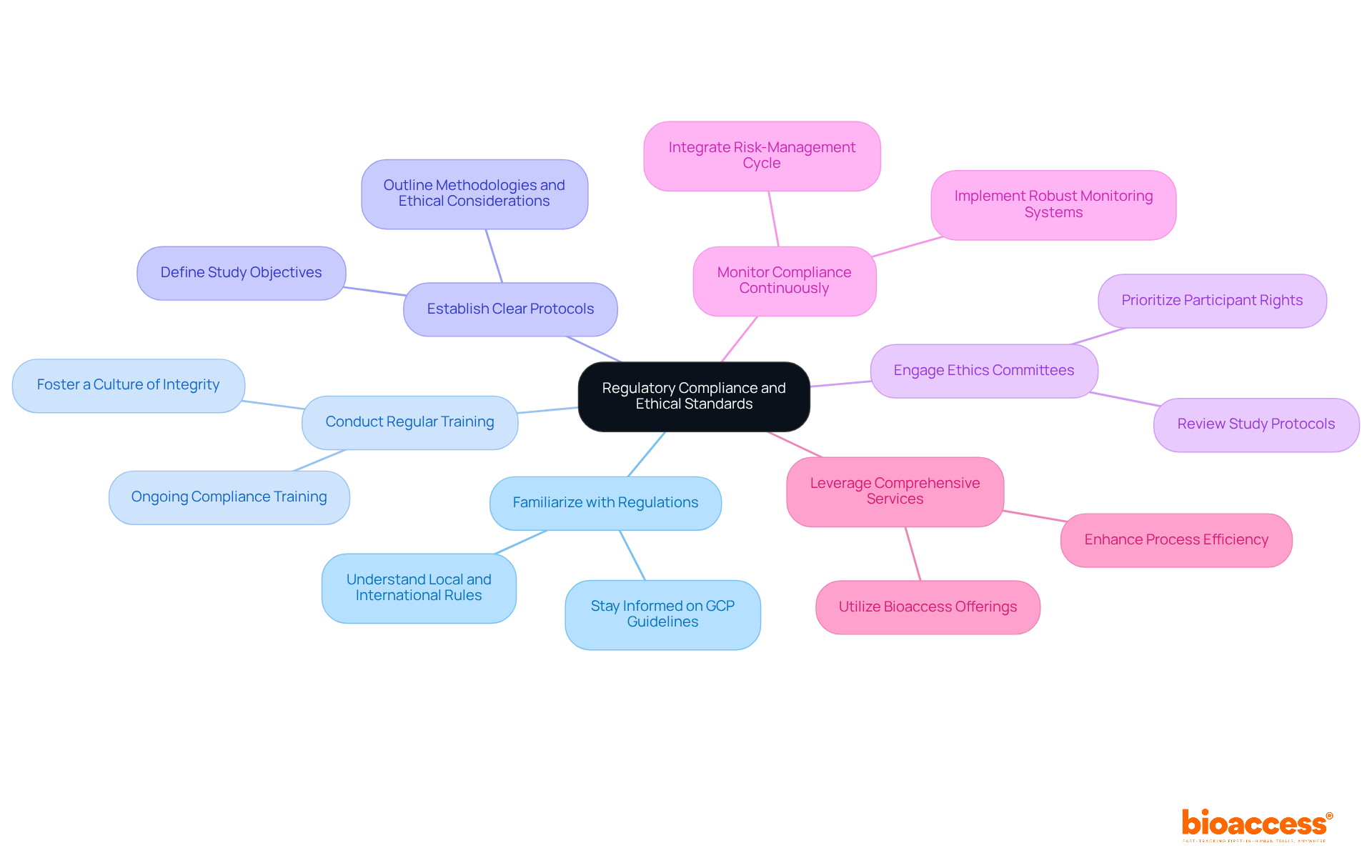
Ensuring regulatory adherence and ethical standards is crucial in research studies. Directors should take decisive steps to navigate these complexities effectively.
Familiarize with Regulations: It is essential to stay informed about local and international rules governing clinical studies, particularly the evolving Good Clinical Practice (GCP) guidelines. Recent updates emphasize a risk-proportionate approach, enhancing participant protection and data integrity.
Conduct Regular Training: Implementing ongoing training for all team members on compliance and ethical considerations fosters a culture of integrity. This ensures that everyone is prepared to maintain the highest standards throughout the research process.
Establish Clear Protocols: Creating and following well-defined guidelines that outline the study's objectives, methodologies, and ethical considerations is vital. A structured protocol promotes clarity and ensures that all team members understand their roles and responsibilities.
Engage Ethics Committees: Collaborating with ethics committees to review study protocols is crucial for prioritizing participant rights and welfare. This engagement is essential for maintaining ethical standards and fostering trust in the research process.
Monitor Compliance Continuously: Implementing a robust monitoring system is necessary to ensure ongoing adherence throughout the study. Regular assessments and prompt resolution of any issues are vital for maintaining the integrity of the study and safeguarding participant welfare. Integrating the six-step risk-management cycle introduced in ICH E6 R3—identifying, evaluating, controlling, communicating, reviewing, and reporting risks—into this monitoring process is essential.
Leverage Comprehensive Services: Utilizing bioaccess's offerings, which include feasibility studies, site selection, compliance reviews, experiment setup, import permits, project management, and reporting, can assist in navigating the complexities of regulatory challenges. These services significantly improve the overall efficiency of the process.
By following these strategies, directors can greatly enhance the success probability of research studies while upholding ethical standards that safeguard participants and guarantee trustworthy data.
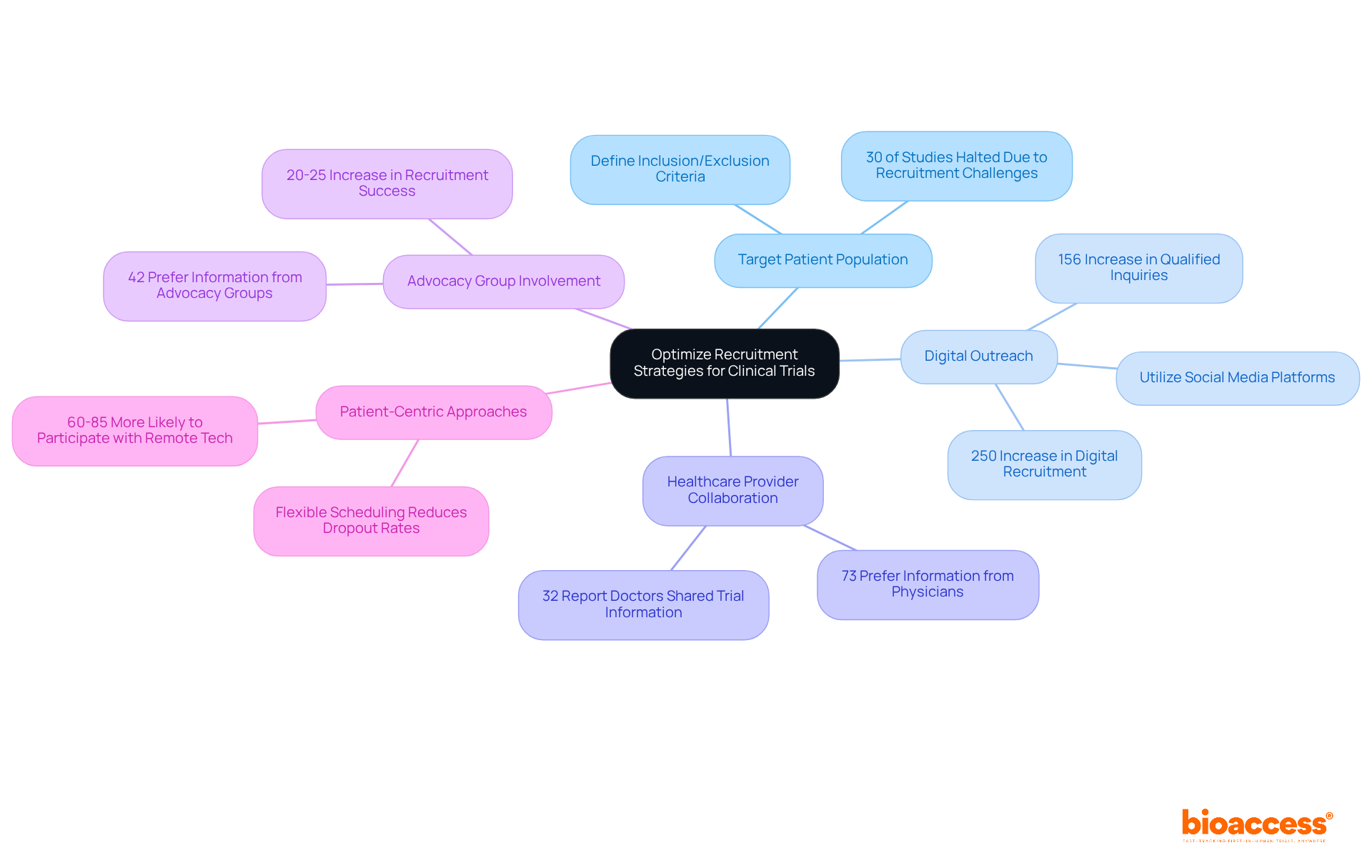
Enhancing recruitment approaches is essential for ensuring that studies are sufficiently robust and completed on time. Directors can enhance recruitment by clearly defining the target patient population based on inclusion and exclusion criteria, which streamlines recruitment efforts. This method is crucial, as nearly 30% of research studies are halted due to recruitment challenges, often stemming from stringent eligibility standards. Furthermore, up to 40% of clinical research sites withdraw before finishing recruitment, underscoring the broader challenges in this field.
Utilizing digital platforms, such as social media and online registries, enables effective outreach to potential participants. With over 3 billion active users on platforms like Facebook and Instagram, digital recruitment has surged by over 250% in the past five years, significantly enhancing engagement and diversity within participant pools.
Establishing connections with healthcare professionals is another critical strategy. Collaborating with nearby healthcare providers raises awareness about studies and promotes referrals. Research indicates that 73% of individuals prefer receiving information about clinical trial opportunities from their physicians, yet only 32% reported that their doctors had ever communicated details about clinical trials to them. This gap highlights the importance of these partnerships.
Involving healthcare advocacy groups can further improve outreach and foster trust within communities. Collaborations with advocacy organizations have been shown to enhance recruitment success probability by 20-25%, making them a valuable asset in recruitment strategies.
Finally, implementing patient-centric approaches is vital. Focusing on patient needs and preferences, such as offering flexible scheduling and minimizing travel burdens, can significantly enhance participation rates. Studies reveal that 60-85% of respondents would be more likely to participate in trials if remote technology reduced travel requirements, emphasizing the critical role of accessibility in recruitment efforts.
In conclusion, boosting the success probability in clinical trials is paramount for advancing medical research and enhancing patient outcomes. By implementing targeted strategies, research directors can significantly elevate their studies' chances of achieving favorable results. This focus on success probability not only facilitates resource allocation but also drives innovation across the Medtech, Biopharma, and Radiopharma sectors.
The article has outlined several key strategies to augment success probability, including:
Furthermore, ensuring regulatory compliance and ethical standards is critical, as these elements directly affect the integrity of the research and the safety of participants. Effective recruitment strategies, such as defining target populations and employing digital platforms, further enhance the overall success of clinical trials.
Ultimately, the significance of these strategies cannot be overstated. By prioritizing success probability and adhering to best practices, clinical trial directors can adeptly navigate the complexities of research while maximizing their chances of achieving meaningful outcomes. Embracing these approaches not only amplifies the potential for scientific breakthroughs but also underscores the commitment to ethical research practices that protect participants and foster trust within the community.
What does success probability mean in clinical trials?
Success probability in clinical trials refers to the likelihood that a study will successfully meet its primary endpoints and demonstrate a favorable risk-benefit profile.
What factors influence success probability in clinical studies?
Success probability is influenced by various factors, including study design, participant demographics, and the specific therapeutic area being investigated.
How do biomarkers affect success probability in clinical trials?
Studies that incorporate biomarkers for patient selection tend to have significantly higher success probabilities of achieving favorable outcomes compared to those that do not use such targeted methods.
What are the typical success probabilities for different phases of clinical trials?
Phase III studies generally have a success probability of around 50%, Phase II studies approximately 30%, and Phase I studies about 60%.
How can understanding success probability benefit research directors?
By clearly outlining success probabilities, research directors can effectively communicate objectives to stakeholders and align their teams towards these goals, optimizing resource allocation and enhancing the likelihood of favorable outcomes.