


The primary objective of this article is to present a structured methodology for selecting the optimal clinical research partner through four essential steps:
Each of these elements is pivotal for the success of clinical trials, reinforcing the critical nature of collaboration in achieving research goals.
Navigating the complex landscape of clinical research requires more than just scientific expertise; it demands the right partnerships. Selecting a clinical research partner can significantly influence the success of a study, impacting everything from regulatory compliance to patient recruitment.
What steps can researchers take to ensure they choose a partner that aligns with their specific objectives and enhances the overall quality of their clinical trials? This guide outlines a structured approach to identifying and evaluating potential collaborators, addressing the critical factors that can make or break a research endeavor.
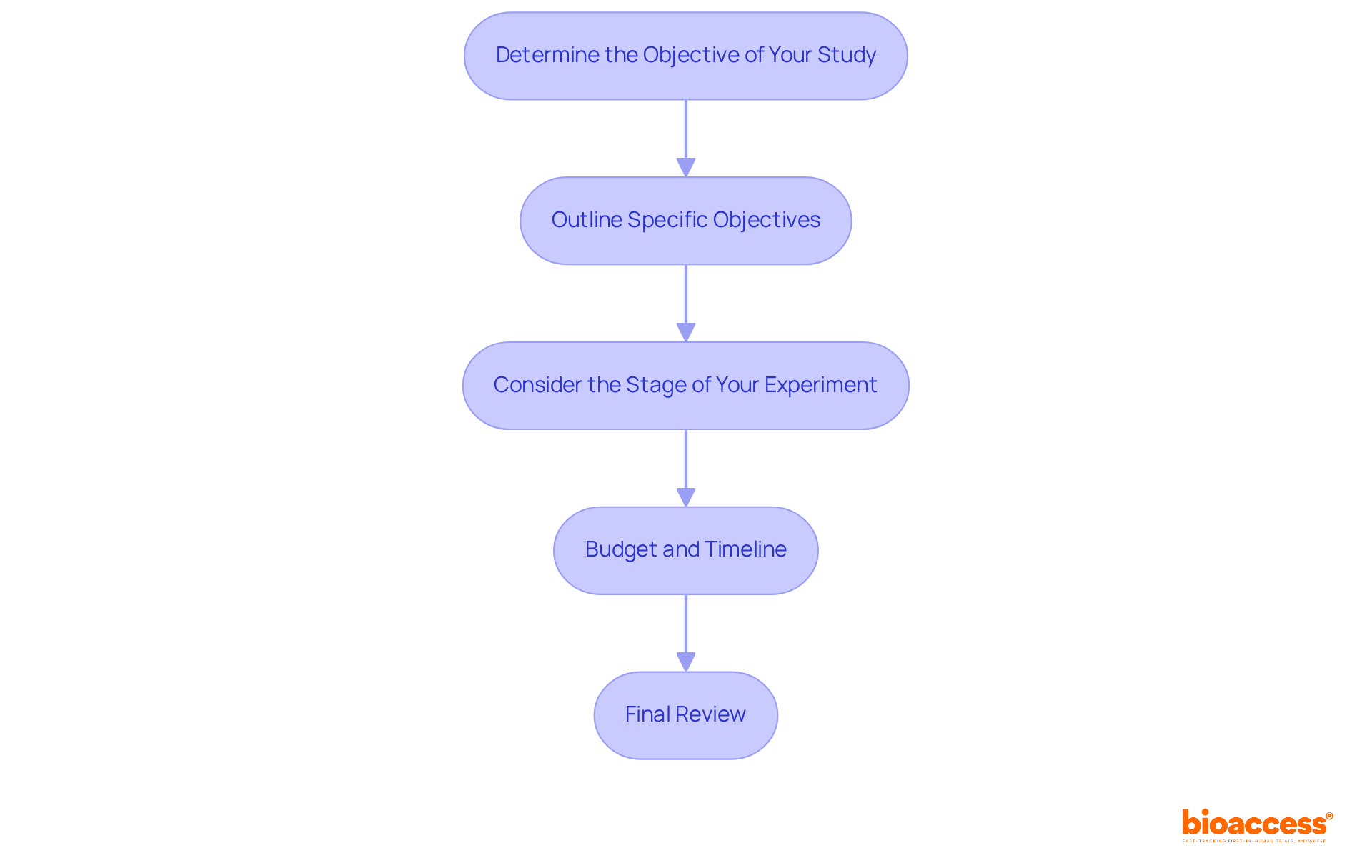
Determine the Objective of Your Study: Begin by clearly defining the primary goal of your research. Are you evaluating a new drug, device, or treatment protocol? A well-articulated purpose not only simplifies the selection of potential collaborators but also enhances the focus when working with your clinical research partner.
Outline Specific Objectives: Establish specific, measurable objectives. For example, if your aim is to assess the efficacy of a new medication, outline the endpoints you will evaluate, such as symptom improvement or reduction in adverse effects. Research indicates that ambiguous goals contribute to approximately 30% of medical study failures, underscoring the critical need for precision in this field.
Consider the Stage of Your Experiment: Different collaborators bring varying expertise across clinical study phases (Phase I, II, III, etc.). Ensure that your objectives align with the capabilities of a clinical research partner. For instance, Phase I studies typically involve a small cohort (10-30 participants) focused on safety, whereas Phase III studies may engage several hundred patients to compare the new treatment against existing standards.
Budget and Timeline: Establish a budget and timeline for your study. This clarity will help you identify collaborators who can operate within your financial and temporal constraints, ensuring that your objectives remain realistic and achievable. The FDA's recent guidance on decentralized studies highlights the importance of flexibility in study design, which can further enhance recruitment and participant diversity.
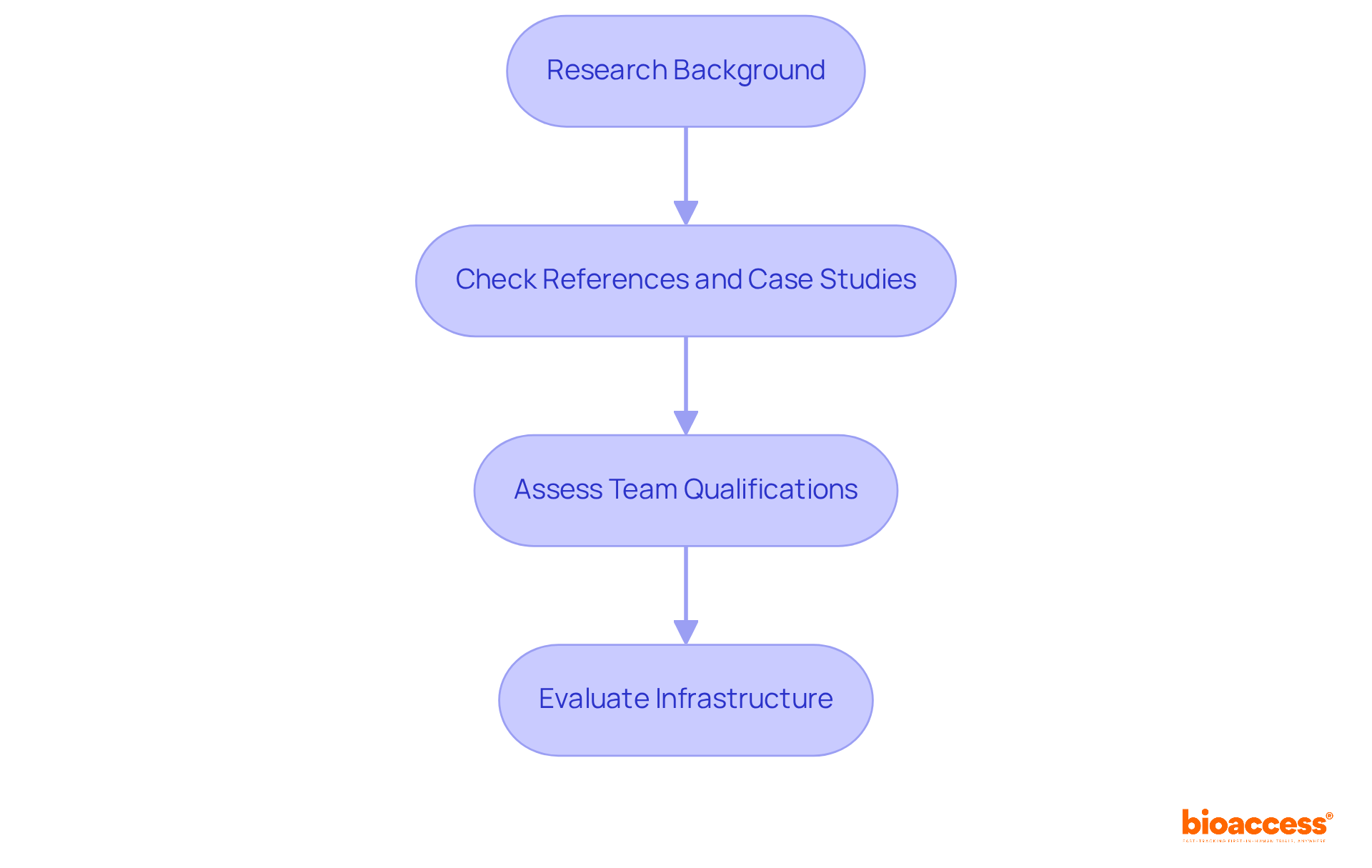
Research their background: Investigate the potential clinical research partner's history in clinical research, focusing on the types of studies they have conducted and their familiarity with your therapeutic area. Understanding their historical performance is essential, as research indicates that a sponsor's success rate in earlier assessments can predict future outcomes.
Check References and Case Studies: Request references from past clients and examine case studies that highlight their success in similar situations. This not only provides insight into their capabilities but also demonstrates their reliability. For instance, collaborations with non-industrial partners have been shown to significantly enhance research success rates.
Assess Their Team's Qualifications: Evaluate the qualifications of the team members who will be involved in your examination. Ensure they possess the necessary certifications and experience as a clinical research partner. A dedicated team with a strong track record can enhance the quality and efficiency of the testing process.
Evaluate Their Infrastructure: Consider the facilities and technology that the partner has in place. A well-equipped collaborator can streamline processes and improve the quality of experimentation. The adoption of advanced technologies, such as blockchain for secure data exchange, has been linked to increased efficiency and integrity in testing, making it vital to assess your collaborator's technological capabilities.
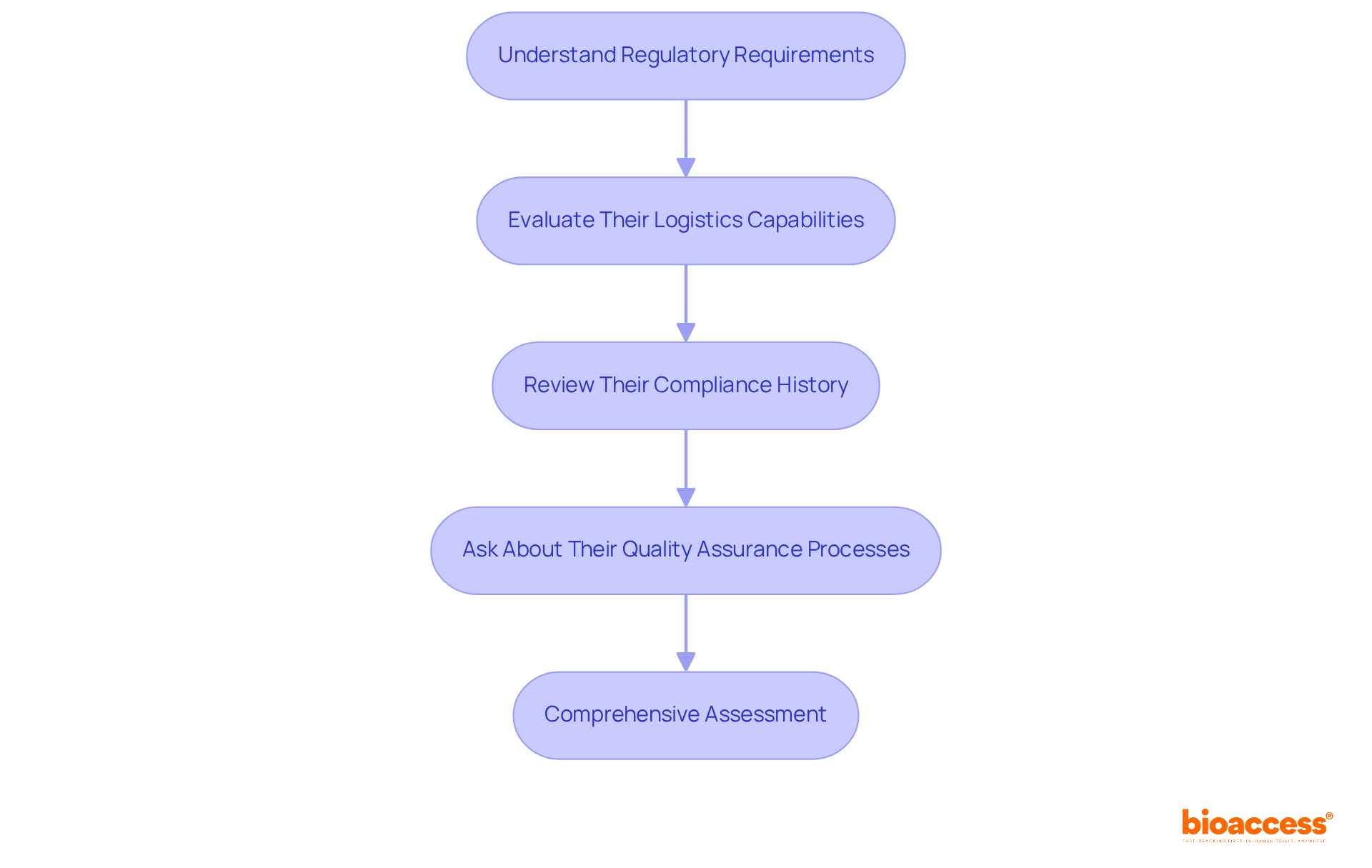
Understand Regulatory Requirements: It is crucial to familiarize yourself with the regulatory landscape specific to your study's location. A clinical research partner must possess a robust understanding of these regulations and demonstrate a consistent history of compliance. Notably, regulatory bodies are increasingly stressing participant diversity in clinical studies, necessitating that collaborators are knowledgeable about these evolving standards.
Evaluate Their Logistics Capabilities: Assess the partner's management of logistics, including patient recruitment, data management, and supply chain operations. Efficient logistics are essential for the prompt execution of tests. In 2022/23, England saw a remarkable increase of over 220,000 participants in clinical studies compared to pre-pandemic levels, underscoring the significance of effective recruitment strategies.
Review Their Compliance History: Investigate any past compliance issues the partner may have encountered. A history of regulatory violations can serve as a warning sign. For instance, frequent non-compliance concerns noted in FDA Warning Letters typically involve protocol deviations and failure to submit Investigational New Drug applications (INDs), which can jeopardize the integrity of the study.
Ask About Their Quality Assurance Processes: Inquire about the quality assurance measures they have implemented to ensure adherence to Good Clinical Practice (GCP) and other relevant standards. The execution of centralized quality management and uniform procedures can significantly enhance data integrity across various locations, as evidenced by recent advancements in research technology. By prioritizing these factors, you can ensure that your research collaborator is well-prepared to navigate the complexities of contemporary studies efficiently.
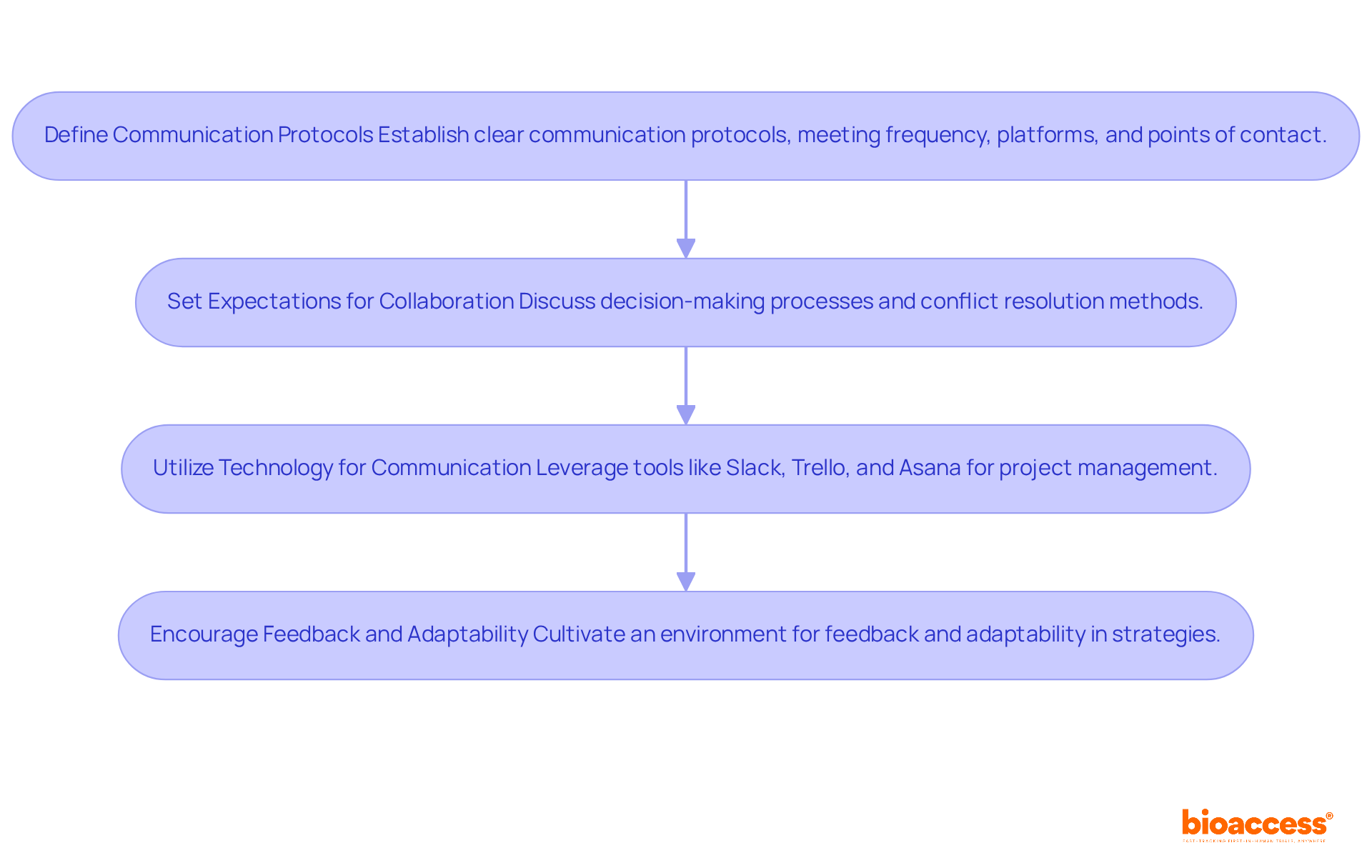
Define Communication Protocols: Establishing clear communication protocols from the outset is crucial. Determine the frequency of meetings, the platforms for communication, and designate primary points of contact to ensure alignment. This is particularly significant in the context of bioaccess's extensive management services for medical testing, which encompass feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, specifically designed for expedited medical device research services in Latin America with a clinical research partner.
Set Expectations for Collaboration: It is essential to discuss and agree on decision-making processes and conflict resolution methods. A cooperative method not only enhances problem-solving but also fosters innovation, as research indicates that varied collaborations significantly boost success rates in medical experiments. This is especially pertinent for the types of research handled by bioaccess, including Early-Feasibility Assessments (EFS) and First-In-Human Trials (FIH).
Utilize Technology for Communication: Leverage modern technology tools for project management and communication. Platforms such as Slack, Trello, and Asana facilitate seamless collaboration, keeping all team members informed and engaged. By 2025, these tools will be essential for improving productivity and ensuring that teams can swiftly adjust to shifts in study dynamics, particularly in the context of expedited medical device research services in Latin America by bioaccess.
Encourage Feedback and Adaptability: Cultivating an environment that encourages feedback is essential. Being open to adapting strategies as the trial progresses allows teams to effectively address challenges. As Helen Keller noted, 'Alone we can do so little; together we can do so much.' This mindset is fundamental in clinical research partnerships, where collaboration can lead to breakthroughs that benefit patients and the healthcare system. Additionally, Patrick Lencioni emphasizes that trust is foundational for effective teamwork, further underscoring the necessity for open communication and collaboration.
Choosing the right clinical research partner is a pivotal step in ensuring the success of any clinical trial. Understanding the specific objectives of the study, evaluating potential partners' expertise, assessing compliance with regulatory standards, and establishing strong communication strategies significantly enhance the likelihood of achieving research goals. The alignment of objectives and capabilities with the right partner not only leads to more efficient trials but also contributes to advancements in healthcare.
Key insights regarding the importance of:
have been highlighted. Each of these elements plays a vital role in the selection process, directly influencing the quality of research outcomes. Furthermore, fostering effective communication and collaboration streamlines processes and promotes innovation, ensuring all parties are aligned toward a common goal.
In light of these considerations, researchers must adopt a proactive approach when selecting a clinical research partner. By meticulously following the outlined steps, stakeholders can mitigate potential risks and enhance the overall effectiveness of their clinical trials. The right partnership paves the way for groundbreaking discoveries that ultimately benefit patients and contribute to the evolution of medical science.
What is the first step in defining clinical trial objectives?
The first step is to clearly define the primary goal of your research, such as evaluating a new drug, device, or treatment protocol.
Why is it important to outline specific objectives in a clinical trial?
Outlining specific, measurable objectives is crucial because ambiguous goals contribute to approximately 30% of medical study failures. Clear objectives enhance focus and simplify collaboration with research partners.
How does the stage of the experiment affect the selection of collaborators?
Different collaborators have varying expertise across clinical study phases (Phase I, II, III, etc.). Your objectives should align with the capabilities of a clinical research partner, as each phase has different focuses and participant numbers.
What should be considered when establishing a budget and timeline for a clinical trial?
Establishing a budget and timeline helps identify collaborators who can work within your financial and temporal constraints, ensuring that your objectives remain realistic and achievable.
What recent guidance has the FDA provided regarding clinical trials?
The FDA's recent guidance on decentralized studies emphasizes the importance of flexibility in study design, which can enhance recruitment and participant diversity.