


As the landscape of clinical research continues to evolve, Colombia has emerged as a noteworthy player in the global arena, aligning its practices with international standards while navigating unique challenges. The role of INVIMA, Colombia's regulatory authority, is paramount in ensuring that clinical trials meet stringent ethical and compliance requirements, thereby safeguarding the integrity of research. Recent collaborations between local entities and international partners are not only accelerating the growth of clinical trials but also enhancing the country’s reputation as a hub for innovative medical research.
This article delves into the current state of clinical research practices in Colombia, comparing them with global standards, examining compliance challenges, and exploring future trends that could redefine the research landscape in the region. Through a comprehensive analysis, insights will be offered into how Colombia's commitment to ethical practices and regulatory excellence is shaping its clinical research ecosystem.
Clinical studies in the country are increasingly aligning with global standards, driven by INVIMA's stringent approval process and oversight. INVIMA plays an essential role in ensuring that studies comply with ethical standards and regulatory obligations, which is crucial for preserving the integrity of investigations carried out in the country. Recent updates indicate that the country has made substantial progress in improving medical study practices, particularly through partnerships like the one between bioaccess™ and Caribbean Health Group, intended to establish Barranquilla as a prominent location for medical evaluations in Latin America. Backed by the Health Minister of the nation, this partnership aims to draw more medical research initiatives to the area.
Furthermore, GlobalCare Clinical Trials (GCCT) has partnered with bioaccess™ to expand its ambulatory services, achieving over a 50% reduction in recruitment time and a retention rate exceeding 95%. This illustrates the country's competitive benefits in executing first-in-human studies, marked by cost efficiency—projected savings of over 30% compared to North America or Western Europe—regulatory speed, and high-quality healthcare.
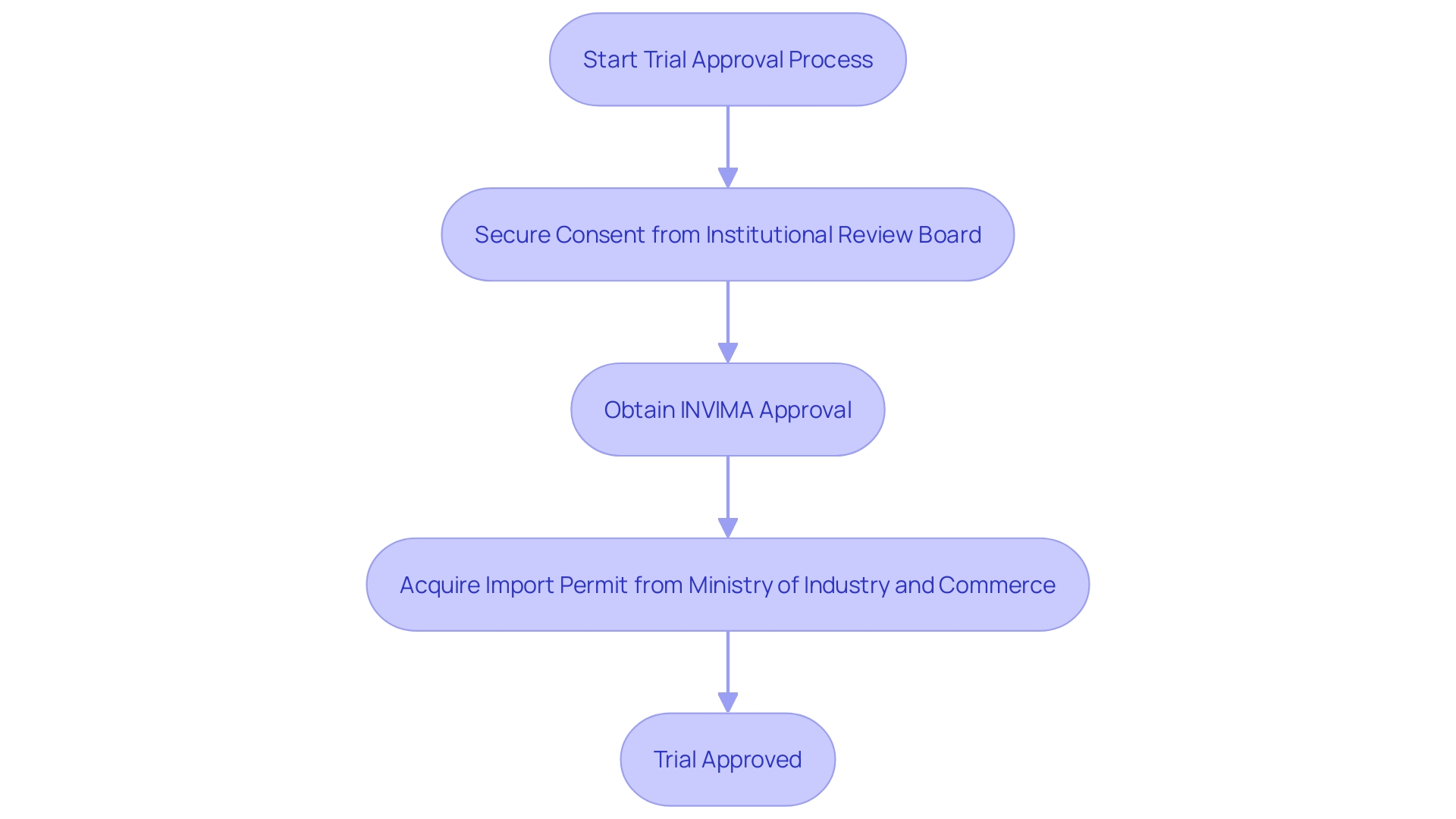
The procedure for gaining trial approval in the country involves several key steps:
These streamlined processes, combined with the country's rich population of over 50 million and universal healthcare coverage for about 95% of citizens, enhance patient recruitment capabilities.
Collaboration between academic institutions and private industry is fostering a more robust innovation ecosystem. However, funding limitations and bureaucratic hurdles often impede progress, highlighting the need for continued investment and policy support. Despite these challenges, the dedication to ethical and compliant medical investigation practices in the country remains strong, contributing to its growing reputation in the global medtech arena. Experts emphasize that ongoing training and adherence to ethical standards are crucial for researchers in Colombia. Overall, INVIMA's role is crucial in shaping the medical study environment, ensuring that evaluations are conducted with the highest standards of integrity and ethical considerations.
Global standards in clinical studies are primarily shaped by the International Council for Harmonization (ICH) Guidelines for Good Clinical Practice (GCP), which emphasize ethical considerations, participant safety, and data integrity. Regulatory bodies such as the FDA and EMA establish stringent criteria for study design and execution, ensuring that research is conducted with the highest ethical standards. The emphasis on transparency, informed consent, and rigorous data monitoring is critical for maintaining public trust and advancing scientific knowledge. Furthermore, global practices promote collaboration across borders, facilitating multi-center studies that enhance diversity and generalizability in research findings.
In contrast, the nation's approach to clinical research is guided by comprehensive management services that include:
The INVIMA, or National Food and Drug Surveillance Institute, plays a vital role in supervising these processes, ensuring that studies adhere to national regulations and ethical standards. Katherine Ruiz, a recognized expert in Regulatory Affairs for medical devices and in vitro diagnostics, has extensive experience advising foreign manufacturers on navigating the Colombian regulatory landscape.
By comparing these standards with those in Colombia, we can observe both alignment and divergence, particularly in areas such as regulatory timelines and ethical review processes. For instance, while both regions emphasize participant safety, the specific requirements for ethical review may differ, impacting the overall timeline for trial initiation. Incorporating case studies that highlight successful collaborations or challenges faced in meeting INVIMA's requirements could further enrich this discussion.
Both Colombian and global research environments face significant compliance challenges, including regulatory delays, ethical review bottlenecks, and difficulties in participant recruitment. In Colombia, bureaucratic inefficiencies can extend approval times, while varying interpretations of ethical guidelines can lead to inconsistencies in study execution. To effectively tackle these challenges, comprehensive clinical study management services are essential. These services include:
A key player in this landscape is the National Food and Drug Surveillance Institute (INVIMA), which oversees medical devices and ensures compliance with health standards. INVIMA is classified as a Level 4 health authority by PAHO/WHO, underscoring its importance in maintaining safety and efficacy in medical products.
Globally, researchers often grapple with multi-jurisdictional regulations, complicating compliance efforts. To mitigate these challenges, effective training and communication within study teams are crucial. Leveraging technology for better data management and reporting can further enhance compliance. Experts like Katherine Ruiz, a Regulatory Affairs authority in medical devices and in vitro diagnostics, offer valuable insights that can help researchers navigate these complexities.
By drawing on best practices from both Colombian and global contexts, researchers can improve compliance and ultimately enhance study outcomes.
The future of medical studies in Colombia is ready for change, especially with the growing adoption of digital health technologies and patient-focused approaches. As telemedicine and remote monitoring become mainstream, Colombian researchers are adapting to these innovations, potentially enhancing recruitment and retention rates.
For example, research has indicated that decentralized medical studies can enhance patient involvement by as much as 30%, illustrating a notable change in engagement approaches. Worldwide patterns like decentralized studies and real-world evidence creation are transforming the investigation landscape, enabling more flexible and adaptive study designs.
Partnerships such as that of Greenlight Guru and bioaccess™ are speeding up Medtech advancements and research in Latin America, illustrated by PAVmed's first-in-human study conducted in the country. As Colombia navigates these changes, aligning with global best practices will be essential to optimize research efficiency and ensure robust outcomes.
Comprehensive trial management services provided by bioaccess®, including:
are essential for addressing existing challenges. Furthermore, these Medtech research studies have a significant impact on local economies, creating jobs—over 1,000 new positions in the past year alone—promoting economic growth, improving healthcare, and fostering international collaboration.
Ultimately, bioaccess® is leading the way in Medtech clinical research in Latin America, focusing on innovation and regulatory excellence to enhance the local sector.
Colombia's clinical research landscape is rapidly evolving, marked by significant advancements that align closely with global standards. The pivotal role of INVIMA in ensuring ethical compliance and regulatory oversight cannot be overstated, as it safeguards the integrity of clinical trials while fostering collaborations that enhance the country’s reputation as a hub for innovative research. The partnerships between local entities and international organizations, such as bioaccess™ and Caribbean Health Group, exemplify the proactive measures being taken to position Colombia as a preferred destination for clinical trials in Latin America.
Despite the challenges posed by bureaucratic inefficiencies and funding limitations, the commitment to maintaining high ethical standards remains strong. The comparison of Colombia's practices with those of global counterparts highlights both alignment and divergence, particularly in regulatory timelines and ethical review processes. This nuanced understanding is essential for navigating the complexities of clinical research, enabling researchers to adopt best practices that enhance compliance and improve study outcomes.
Looking ahead, the integration of digital health technologies and patient-centric methodologies represents a transformative shift in clinical research in Colombia. As the landscape continues to adapt to global innovations, the emphasis on decentralized trials and real-world evidence generation is set to redefine engagement strategies, ultimately enhancing recruitment and retention rates. The ongoing commitment to regulatory excellence and innovative practices will not only bolster Colombia's standing in the global medtech arena but also contribute to economic growth and improved healthcare outcomes for its citizens.
How is INVIMA contributing to clinical studies in Colombia?
INVIMA ensures that clinical studies in Colombia comply with ethical standards and regulatory obligations, which is vital for maintaining the integrity of investigations. The agency's stringent approval process and oversight align local studies with global standards.
What recent partnerships are enhancing medical research in Colombia?
A notable partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a key location for medical evaluations in Latin America. This initiative is supported by the Health Minister and is designed to attract more medical research initiatives to the region.
What improvements have been observed in clinical trial recruitment and retention in Colombia?
GlobalCare Clinical Trials (GCCT) has partnered with bioaccess™ to expand ambulatory services, achieving over a 50% reduction in recruitment time and a retention rate exceeding 95%, showcasing Colombia's competitive advantages in conducting clinical studies.
What are the cost advantages of conducting clinical studies in Colombia?
Conducting clinical studies in Colombia can lead to projected savings of over 30% compared to North America or Western Europe, along with benefits such as regulatory speed and high-quality healthcare.
What are the key steps in the trial approval process in Colombia?
The trial approval process in Colombia involves three main steps: securing consent from the institutional review board (IRB) or ethics committee (EC), obtaining approval from INVIMA, and acquiring an import permit from the Ministry of Industry and Commerce (MinCIT).
How does the population and healthcare coverage in Colombia impact clinical studies?
Colombia has a rich population of over 50 million people and provides universal healthcare coverage for about 95% of its citizens, which enhances patient recruitment capabilities for clinical studies.
What challenges does the Colombian clinical research environment face?
The Colombian clinical research environment faces challenges such as funding limitations, bureaucratic hurdles, regulatory delays, and varying interpretations of ethical guidelines that can impact study execution.
How is the collaboration between academic institutions and private industry affecting innovation in Colombia?
Collaboration between academic institutions and private industry is fostering a more robust innovation ecosystem in Colombia, although challenges like funding limitations remain.
What role does ongoing training play in clinical research in Colombia?
Ongoing training and adherence to ethical standards are crucial for researchers in Colombia to maintain compliance and uphold the integrity of medical investigations.
What future trends are expected to influence medical studies in Colombia?
The adoption of digital health technologies and patient-focused approaches, such as decentralized medical studies, is expected to enhance patient involvement and improve recruitment and retention rates in clinical research.