


This article evaluates device vigilance training providers in Mexico, scrutinizing their offerings, strengths, and weaknesses within the realm of medical device safety and compliance. It is crucial to recognize the significance of regulatory adherence, effective training content, and provider reputation. These elements are instrumental in fostering improved patient safety and compliance, especially as the medical equipment market continues to expand.
Understanding the Medtech landscape and the challenges it presents highlights the essential role that these providers play in addressing key issues. Collaboration among stakeholders is imperative for the advancement of safety standards and compliance measures.
As we delve deeper into the subject, it becomes evident that enhancing training programs can lead to substantial benefits in patient outcomes and regulatory alignment. The next steps involve a concerted effort to engage with these providers and assess their contributions to the field.
The landscape of medical device vigilance training in Mexico is undergoing rapid evolution, propelled by escalating regulatory demands and a burgeoning market projected to reach $137.61 billion by 2029. As healthcare organizations endeavor to enhance patient safety and ensure compliance, the selection of an appropriate training provider becomes crucial. This article conducts a comparative analysis of leading device vigilance training providers, elucidating their strengths, weaknesses, and distinctive offerings.
What essential factors should organizations weigh when choosing the right provider to guarantee effective training and compliance in an environment characterized by increasing medical equipment recalls and heightened regulatory scrutiny?
Monitoring awareness education is essential for the continuous supervision of medical instruments, ensuring their safety and efficacy throughout their lifecycle. This program addresses critical regulatory requirements, the process for reporting adverse events, and the implementation of corrective actions when necessary. The scope of equipment oversight education extends beyond mere technical supervision to encompass the ethical responsibilities related to patient safety. As the medical equipment market expands, particularly in Mexico, the role of a device vigilance training provider in awareness education becomes increasingly apparent. Evolving regulatory frameworks are designed to enhance patient protection and equipment efficacy, making it imperative for professionals to be well-prepared to navigate these complexities.
Effective development fosters a culture of safety and compliance within organizations, ultimately leading to improved patient outcomes and a more resilient healthcare system. Notably, the medical equipment vigilance market is projected to reach $137.61 billion by 2029, expanding at a CAGR of 8.9%, which emphasizes the increasing demand for device vigilance training providers in Mexico. Furthermore, the FDA has reported an increase in medical equipment recalls, with 442 recorded in 2022, highlighting the necessity for comprehensive education.
A course on Medical Device Vigilance is scheduled for September 15-16, 2025, providing an opportunity for professionals to enhance their skills in this critical area. Real-world examples of effective equipment supervision programs illustrate the practical benefits of such education, emphasizing its vital role in ensuring patient safety.
When assessing device vigilance training providers, several essential criteria should be prioritized:
Regulatory compliance mandates that providers must exhibit a comprehensive understanding of both local and international regulations governing the role of a device vigilance training provider in Mexico, particularly the COFEPRIS guidelines. This compliance is crucial, as 95% of organizations are actively building a culture of compliance, underscoring its importance in the industry.
Training Content: The curriculum should encompass vital topics such as adverse event reporting, risk management, and post-market surveillance. Effective development programs are associated with enhanced compliance results, with only 10% of employees indicating that compliance education has influenced their work practices, emphasizing the necessity for more efficient instructional content.
Instructor Expertise: Trainers should have relevant industry experience and qualifications, ensuring they can offer practical insights and real-world applications. Organizations with knowledgeable trainers are better equipped to navigate complex regulatory landscapes, reinforcing the importance of this criterion.
Flexibility and Accessibility: Educational formats must cater to diverse learning styles and schedules, providing options like online courses, in-person workshops, and customized learning solutions. With 89% of employees favoring learning that is accessible anytime and anywhere, flexibility is crucial for engagement.
Feedback and Evaluation: Efficient educators should establish systems for participant feedback and evaluation to measure instructional effectiveness and encourage ongoing enhancement. Significantly, 44% of organizations fail to evaluate the effectiveness of their policy management program, highlighting a gap that efficient educators should address.
Reputation and Track Record: Examining the organization's background, client feedback, and success narratives can provide essential insights into their dependability and efficiency in delivering quality education. A strong reputation often correlates with successful educational outcomes, making this criterion essential for assessment.
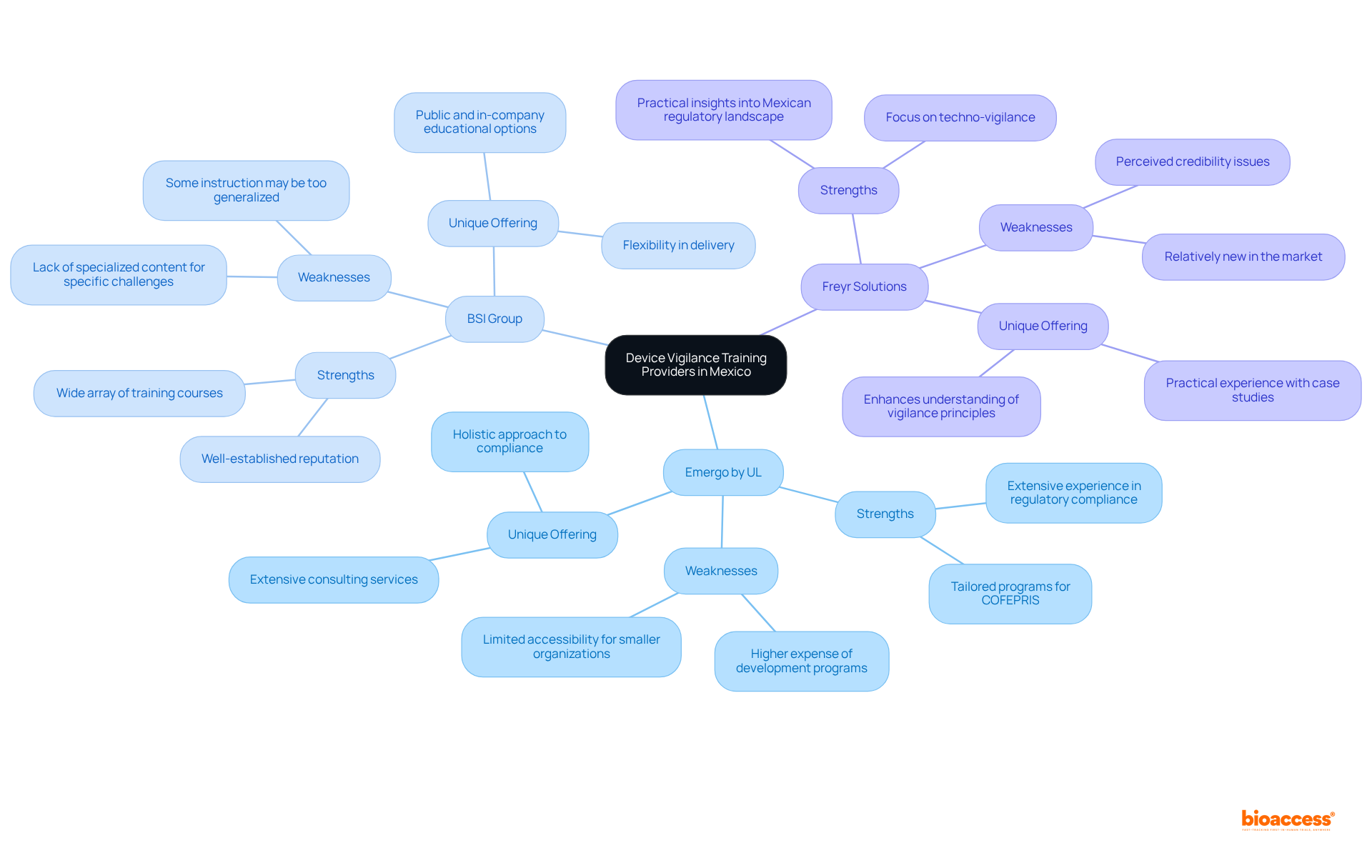
In Mexico, the device vigilance training provider Mexico offers several distinct services to cater to the medical device industry. This analysis compares three leading providers:
Emergo by UL:
BSI Group:
Freyr Solutions:
This comparative analysis underscores the diverse offerings available in Mexico, allowing organizations to choose a device vigilance training provider Mexico that best aligns with their specific training needs and regulatory compliance objectives. The Medical Equipment Vigilance Market is anticipated to expand considerably, reaching USD 133.83 billion by 2032, driven by the increasing occurrence of chronic illnesses and medical equipment adverse events. As noted by industry specialists, "Increasing occurrence of chronic illnesses and a rise in medical equipment adverse events are significant factors propelling the expansion of the global medical equipment monitoring market." This context emphasizes the critical importance of effective awareness preparation in ensuring compliance and enhancing patient safety.
Selecting the appropriate device vigilance training provider in Mexico is essential for ensuring compliance and improving patient safety. To guide your decision-making process, consider the following recommendations and insights:
Assess Organizational Needs: Begin by evaluating your organization's specific development requirements, including the level of expertise needed and the regulatory landscape you operate within. A thorough assessment can lead to a more effective development strategy, as 59% of the workforce believes that such programs enhance job performance.
Research Suppliers Thoroughly: Investigate potential suppliers by reviewing their course offerings, instructor qualifications, and client testimonials. Seek suppliers with a demonstrated history in the medical device sector; this experience can significantly influence the quality of education.
Explore Personalization Alternatives: Choose sources that offer adjustable educational solutions designed to meet your organization's specific requirements and obstacles. Customization ensures that the development is pertinent and applicable, particularly as 38% of employees anticipate learning relevant to their job roles.
Assess Post-Instruction Assistance: Reflect on whether the supplier offers continued support or resources after the instruction is concluded. Continuous support can reinforce learning and help address any subsequent questions or challenges that arise.
Seek Suggestions: Utilize industry networks and professional associations to collect recommendations and insights from colleagues who have previously interacted with educational facilitators. This can provide valuable perspectives on provider effectiveness.
Pilot Programs: If feasible, consider including a small group in a pilot program to evaluate the program's effectiveness before making a larger investment. This approach enables organizations to assess the program's impact on their specific needs.
By adhering to these suggestions, organizations can make informed decisions that enhance their efforts with the device vigilance training provider in Mexico. Notably, companies that offer comprehensive training programs have 218% higher income per employee than those without formalized training, ultimately contributing to improved patient safety and regulatory compliance.
The significance of device vigilance training in the medical sector is paramount, particularly in a rapidly evolving market like Mexico. Ensuring that professionals possess the necessary knowledge and skills to monitor medical equipment effectively is crucial for enhancing patient safety and regulatory compliance. As the demand for vigilance training providers increases, organizations must carefully select partners that align with their specific needs and regulatory requirements.
This article highlights essential evaluation criteria for device vigilance training providers, including:
By comparing leading providers such as Emergo by UL, BSI Group, and Freyr Solutions, it becomes evident that each offers unique strengths and weaknesses. This allows organizations to make informed choices based on their particular circumstances. Furthermore, the recommendations stress the importance of thorough research, personalization of training solutions, and post-instruction support, collectively contributing to a more effective training experience.
Ultimately, investing in device vigilance training transcends mere regulatory obligation; it is a vital component of fostering a culture of safety and compliance in healthcare. Organizations are encouraged to prioritize this training to improve patient outcomes and operational efficiency. By selecting the right training provider, medical institutions can enhance their vigilance capabilities, thereby contributing to a safer healthcare environment and better management of medical device incidents. As the medical equipment vigilance market continues to expand, proactive engagement in training will be key to navigating the complexities ahead.
What is the purpose of device vigilance training?
Device vigilance training is essential for the continuous supervision of medical instruments, ensuring their safety and efficacy throughout their lifecycle. It addresses regulatory requirements, reporting adverse events, and implementing corrective actions when necessary.
Why is monitoring awareness education important in the medical equipment field?
Monitoring awareness education is important because it encompasses not only technical supervision but also the ethical responsibilities related to patient safety. It helps professionals navigate evolving regulatory frameworks designed to enhance patient protection and equipment efficacy.
What is the projected growth of the medical equipment vigilance market?
The medical equipment vigilance market is projected to reach $137.61 billion by 2029, expanding at a compound annual growth rate (CAGR) of 8.9%.
How many medical equipment recalls were reported by the FDA in 2022?
The FDA reported 442 medical equipment recalls in 2022, highlighting the necessity for comprehensive education in device vigilance.
When is the upcoming course on Medical Device Vigilance scheduled?
A course on Medical Device Vigilance is scheduled for September 15-16, 2025, offering professionals an opportunity to enhance their skills in this critical area.
What are the benefits of effective equipment supervision programs?
Effective equipment supervision programs foster a culture of safety and compliance within organizations, leading to improved patient outcomes and a more resilient healthcare system. Real-world examples illustrate the practical benefits of such education.