


The article provides essential insights for directors comparing US Clinical Research Organizations (CROs), facilitating their selection process. It underscores the critical importance of evaluating factors such as:
By ensuring that selected CROs align with the specific needs and objectives of clinical studies, the article aims to enhance research efficiency and success rates.
The landscape of clinical research is rapidly evolving, placing US Clinical Research Organizations (CROs) at the forefront of this transformation. These specialized entities not only streamline the complex processes of drug development but also play a critical role in ensuring compliance and enhancing study efficiency. As the demand for innovative therapies grows, so does the necessity for directors to effectively navigate the diverse offerings of CROs. Understanding the key factors that can make or break the selection of the right CRO is crucial. How can these insights lead to more successful clinical trials?
Us clinical research organizations (CROs) are specialized entities that deliver a comprehensive range of services essential for the planning, execution, and management of clinical studies. Us clinical research organizations play a vital role in the drug development process, offering expertise in regulatory compliance, patient recruitment, data management, and study monitoring. The diversity among CROs in terms of size, scope, and specialization enables them to cater to various sectors, including pharmaceuticals, biotechnology, and medical devices.
The primary objective of CROs is to optimize the clinical study process, ensuring that investigations are conducted effectively, ethically, and in alignment with regulatory standards. This goal is particularly significant as the global market for CROs is projected to expand considerably, driven by an increasing number of new drug approvals and the demand for personalized medicine. By 2025, CROs are expected to enhance research study success rates, addressing the challenges posed by complex study designs and recruitment difficulties.
Statistics indicate that medical studies account for nearly 40% of the US pharmaceutical research budget, totaling approximately $7 billion annually. However, around 80% of research studies fail to meet initial enrollment targets, leading to substantial financial losses estimated at $8 million per day for drug development firms. This underscores the critical need for effective patient recruitment strategies, an area in which us clinical research organizations excel.
Illustrative case studies reveal how CROs have significantly improved study management. For example, bioaccess®, a US-based CRO with over two decades of experience in Medtech, has proven its ability to facilitate research for Medtech startups in Latin America, focusing on delivering fast, cost-effective, and high-quality data through various studies, including:
A notable collaboration between GlobalCare Clinical Trials and bioaccess® enhanced ambulatory services for research in Colombia, achieving over a 50% reduction in recruitment time and a 95% retention rate. This partnership highlights the effectiveness of CROs in improving study efficiency and participant engagement.
Understanding the capabilities and alignment of potential us clinical research organizations partners is essential for directors when assessing their options. The expertise that CROs provide not only enhances the effectiveness of clinical studies but also mitigates risks associated with delays and compliance challenges, ultimately facilitating the successful advancement of new therapies.
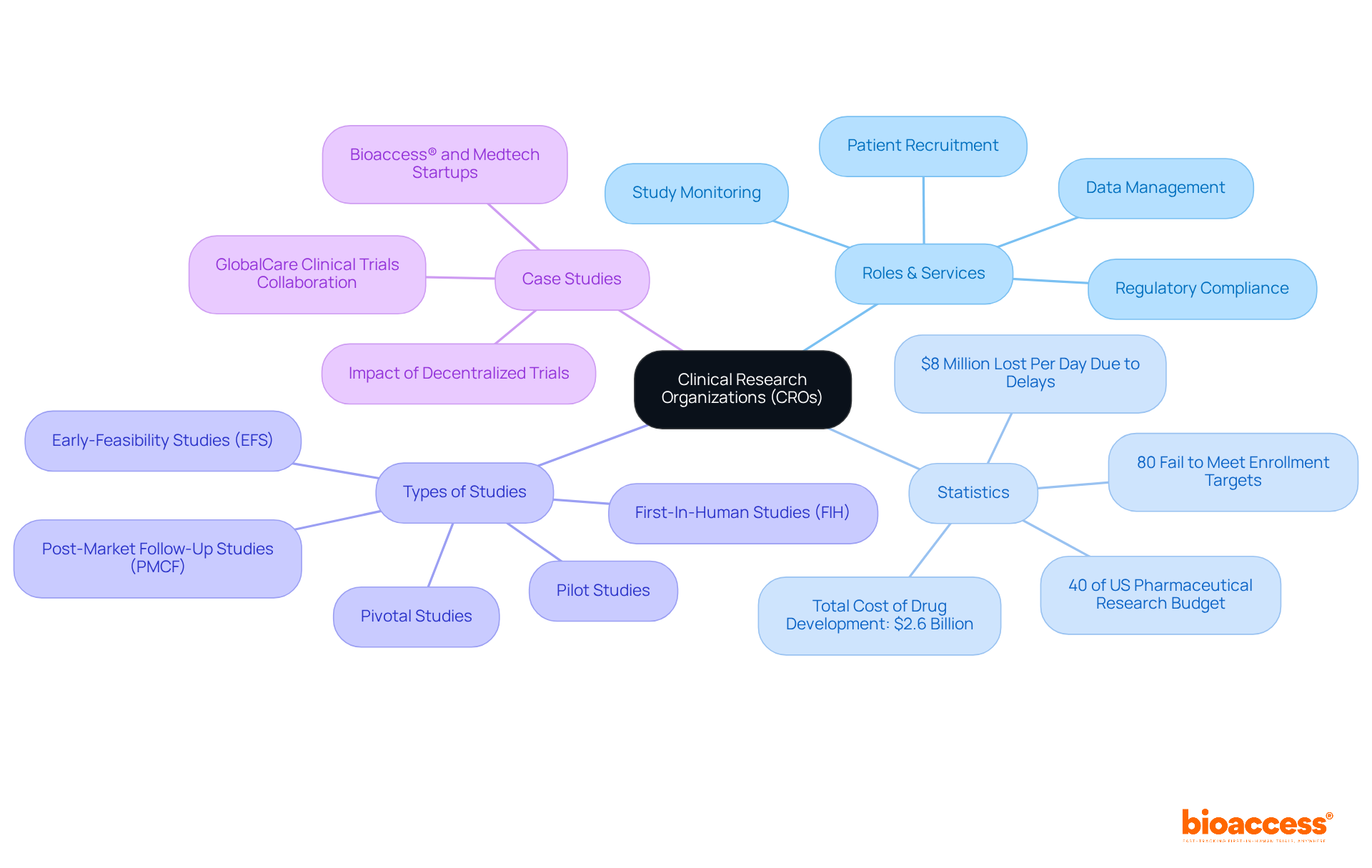
When selecting a CRO, directors must consider several key criteria:
Experience and Expertise: Evaluate the CRO's background in overseeing studies that correspond with your project, especially in pertinent therapeutic areas and stages of development. A CRO with a proven track record can significantly impact study outcomes.
Regulatory Knowledge: A deep understanding of the regulatory landscape is crucial. Ensure the CRO is well-versed in both local and international guidelines, as this knowledge directly impacts compliance success rates. For example, CROs that excel in regulatory affairs often report higher approval rates and fewer delays in study progression.
Technology and Innovation: Evaluate the CRO's technological capabilities, including advanced data management systems and patient recruitment tools. The integration of innovative technologies can enhance testing efficiency and data integrity, which are vital for successful outcomes.
Geographic Reach: Consider the CRO's capacity to carry out studies in the required locations, particularly if your research necessitates access to varied patient populations. A CRO with a broad geographic footprint can facilitate recruitment and ensure a representative sample.
Cost and Value: Analyze the CRO's pricing structure alongside the value they provide. It's essential to ensure that their services align with your budget while delivering quality outcomes. Understanding the cost-benefit ratio can help in making informed decisions.
Communication and Collaboration: Assess the CRO's communication approach and their readiness to work closely with your team during the process. Effective communication is key to navigating challenges and ensuring that all stakeholders are aligned.
These standards offer a strong structure for directors to efficiently evaluate potential US clinical research organizations as partners, guaranteeing that the selected organization corresponds with the specific requirements and objectives of their research projects.
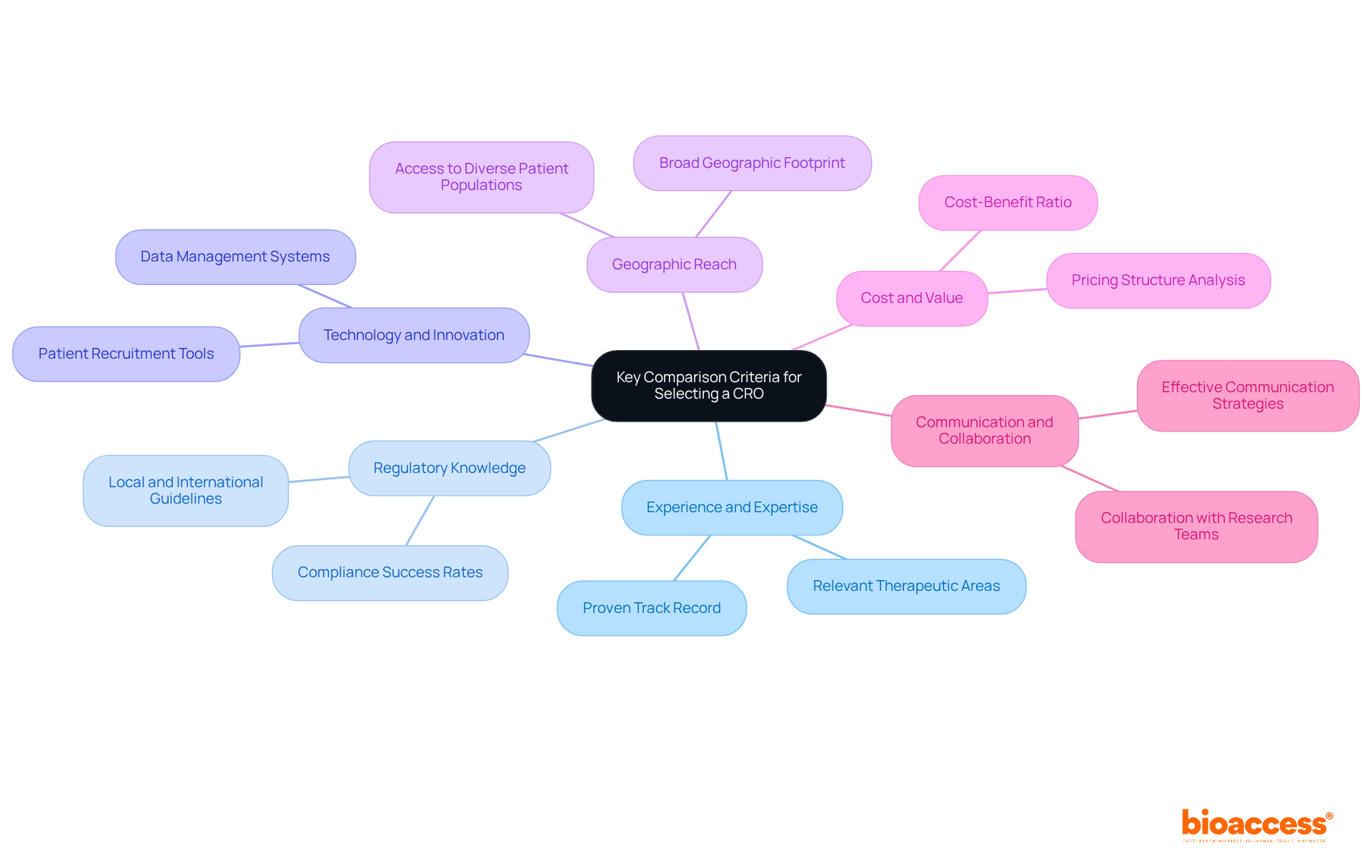
In 2025, several US clinical research organizations, including IQVIA, Parexel, Medpace, and bioaccess, distinguish themselves in the market.
IQVIA: Recognized for its data-oriented approach, IQVIA excels in combining advanced analytics and technology into research studies. With an extensive global reach and a robust patient database, it is the preferred choice for large-scale studies, enabling efficient data collection and analysis.
Parexel: Parexel stands out with its strong emphasis on regulatory compliance and patient-centric solutions. Its expertise in navigating complex regulatory landscapes is complemented by comprehensive service offerings, including market access strategies that are invaluable for clients aiming to commercialize their products successfully. Success stories highlight Parexel's ability to streamline the regulatory process, ensuring timely approvals and market entry.
Medpace: Recognized for its therapeutic knowledge and operational effectiveness, Medpace excels in early-phase studies. Its integrated method ensures that clients obtain customized solutions aligned with their specific research goals, improving the overall effectiveness of studies.
bioaccess: bioaccess provides an extensive range of management services for research studies, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting. Their focus on international collaboration and innovation in medtech positions them as a key player in driving global health improvement.
This comparative analysis underscores the strengths and unique offerings of each CRO, equipping directors with critical insights to inform their selection process. The market size of US clinical research organizations is projected to reach $24.1 billion in 2025, emphasizing the significance of these leading organizations in a rapidly evolving landscape.
Selecting the appropriate CRO necessitates a strategic method that aligns with the specific requirements of your clinical study. Directors must begin by clearly defining project objectives, which include timelines, budget constraints, and desired outcomes. This foundational step ensures that all stakeholders are aligned on expectations and deliverables.
Thorough research on potential US clinical research organizations is essential. Evaluation standards should encompass their history in managing project timelines and compliance with budgets, as these elements greatly influence success. Engaging in discussions with shortlisted US clinical research organizations provides valuable insights into their operational processes and cultural fit, both of which are crucial for fostering a productive partnership.
Additionally, seeking feedback from previous clients illuminates the CRO's reliability and performance, offering a clearer picture of their capabilities. Successful partnerships often hinge on effective communication and collaboration, enhancing the overall research experience.
Ultimately, the ideal CRO will not only meet the technical requirements of the trial but also cultivate a collaborative environment that supports research objectives, ensuring a smoother path to successful outcomes.

The role of US clinical research organizations (CROs) is pivotal in the landscape of drug development, providing essential services that enhance the execution and management of clinical studies. With the industry poised for significant growth by 2025, understanding the unique capabilities of various CROs becomes crucial for directors aiming to optimize their research efforts. By leveraging the expertise of CROs, organizations can navigate the complexities of clinical trials more effectively, ensuring compliance and ultimately, the success of new therapies.
Key insights from the article highlight the importance of selecting a CRO based on specific criteria, including:
The comparative analysis of leading CROs such as IQVIA, Parexel, Medpace, and bioaccess illustrates how each organization brings distinct strengths to the table, enabling directors to make informed decisions that align with their project needs. Furthermore, the article emphasizes the necessity of thorough research and collaboration in establishing successful partnerships, which are vital for driving positive outcomes in clinical trials.
Ultimately, the significance of CROs in the clinical research ecosystem cannot be overstated. As the demand for innovative therapies continues to rise, the ability to choose the right CRO will play a critical role in advancing research objectives. Directors are encouraged to adopt a strategic approach in their selection process, ensuring that the chosen organization not only meets technical requirements but also fosters an environment of collaboration and support. By doing so, they can enhance their chances of success in an increasingly competitive landscape.
What are Clinical Research Organizations (CROs)?
Clinical Research Organizations (CROs) are specialized entities that provide a comprehensive range of services necessary for the planning, execution, and management of clinical studies, playing a vital role in the drug development process.
What services do CROs offer?
CROs offer expertise in regulatory compliance, patient recruitment, data management, and study monitoring, among other services.
Why are CROs important in drug development?
CROs optimize the clinical study process, ensuring investigations are conducted effectively, ethically, and in compliance with regulatory standards, which is crucial for the success of new drug approvals and personalized medicine.
What is the projected growth of the CRO market?
The global market for CROs is expected to expand considerably by 2025, driven by an increase in new drug approvals and the demand for personalized medicine.
What challenges do CROs help address in clinical studies?
CROs help enhance research study success rates and address challenges such as complex study designs and difficulties in patient recruitment.
How significant is the financial impact of research study failures?
Approximately 80% of research studies fail to meet initial enrollment targets, leading to financial losses estimated at $8 million per day for drug development firms.
Can you provide an example of a successful CRO?
Bioaccess®, a US-based CRO, has over two decades of experience in Medtech and has facilitated research for Medtech startups in Latin America, delivering fast, cost-effective, and high-quality data through various studies.
What types of studies do CROs conduct?
CROs conduct a variety of studies including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
How do CROs improve recruitment and retention in studies?
Collaborations, such as that between GlobalCare Clinical Trials and bioaccess®, have demonstrated significant improvements, including over a 50% reduction in recruitment time and a 95% retention rate.
What should directors consider when choosing a CRO?
Directors should assess the capabilities and alignment of potential CRO partners to ensure they enhance the effectiveness of clinical studies and mitigate risks related to delays and compliance challenges.