


The article provides a comprehensive comparison of clinical research organizations (CROs) in Europe, underscoring their vital roles, strengths, and selection criteria. It asserts that effective collaboration with CROs, such as bioaccess®, which is renowned for its rapid patient recruitment and regulatory approvals, is essential for the success of clinical studies. This is particularly significant as the demand for innovative treatments continues to escalate within the pharmaceutical and biotech sectors. By highlighting these insights, the article emphasizes the necessity of choosing the right CROs to navigate the complexities of clinical research effectively.
Clinical research organizations (CROs) in Europe play a pivotal role in the pharmaceutical and biotechnology sectors, acting as essential partners in the development of new therapies. As the demand for efficient and effective clinical trials continues to grow, understanding the diverse landscape of CROs becomes crucial for companies aiming to navigate complex regulatory environments and optimize study processes.
However, with numerous options available, how can organizations determine which CRO aligns best with their specific needs and goals? This article delves into key insights and comparative analyses of leading CROs in Europe, providing a roadmap for selecting the right partner in the evolving world of clinical research.
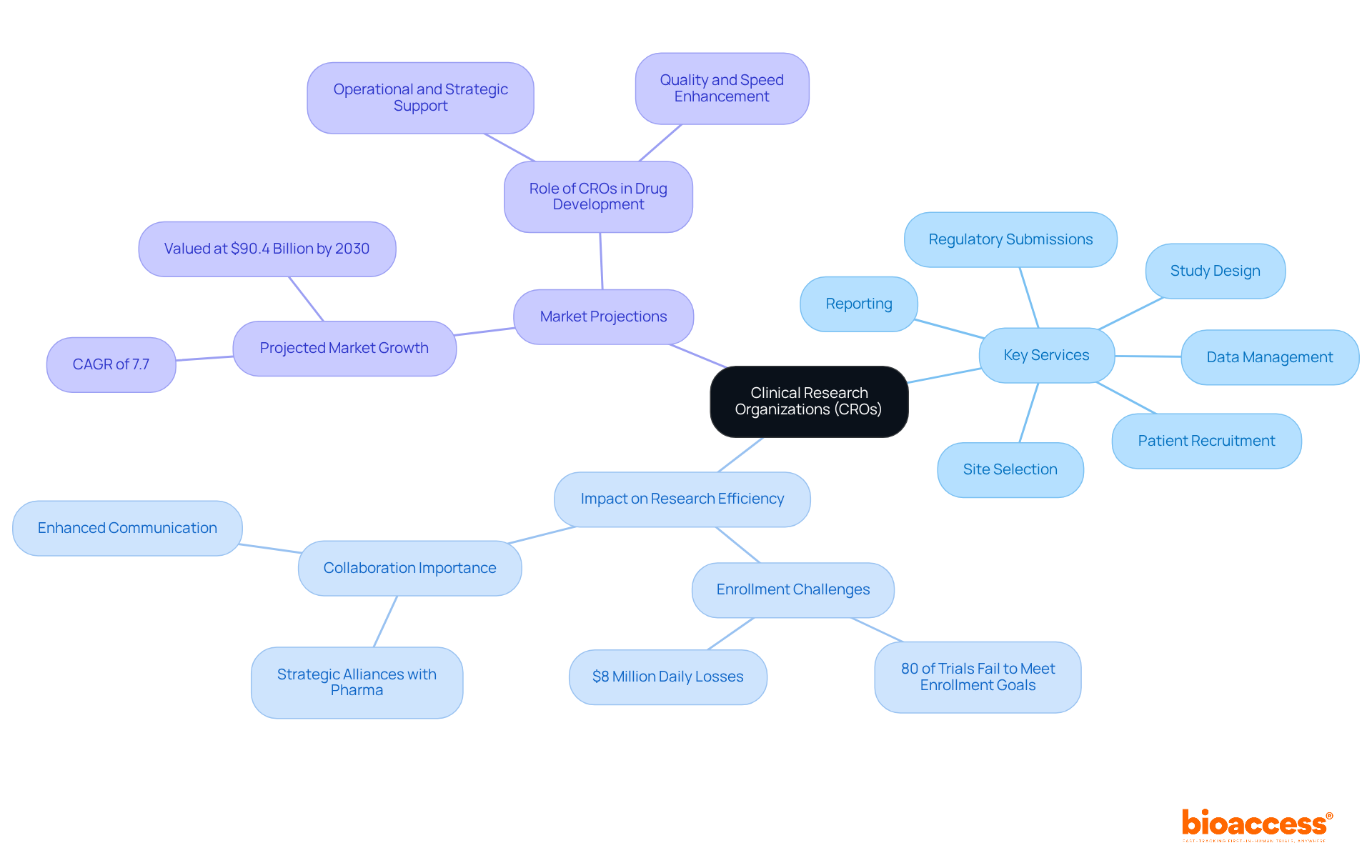
Clinical research organizations in Europe play a crucial role in the pharmaceutical, biotechnology, and medical device industries by offering outsourced research services that optimize the study process. Their expertise includes managing trials, ensuring regulatory compliance, and facilitating data collection and analysis, all of which are essential for the successful development of new therapies. Contract research organizations, which include clinical research organizations in Europe, vary significantly in size, scope, and specialization, with many focusing on specific therapeutic fields or stages of clinical research. Key services provided by CROs encompass:
This comprehensive suite of services positions CROs as invaluable partners for companies aiming to bring innovative therapies to market efficiently.
The influence of CROs on research study efficiency is substantial. For instance, approximately 80% of medical studies fail to meet initial enrollment targets, resulting in significant financial losses estimated at $8 million daily for pharmaceutical development firms. By leveraging their expertise and resources, clinical research organizations in Europe can enhance patient recruitment strategies, thereby mitigating these risks. Moreover, successful collaborations between clinical research organizations in Europe and pharmaceutical firms are increasingly recognized as a vital element in achieving trial success. Industry leaders emphasize that effective cooperation can yield better results and faster timelines, underscoring the importance of CROs in the evolving landscape of medical research.
As we look towards 2025, the role of Chief Research Officers continues to expand, with the global market for contract research outsourcing projected to reach $90.4 billion by 2030, reflecting a compound annual growth rate of 7.7%. This growth is driven by the increasing reliance of pharmaceutical and biotech firms on clinical research organizations in Europe to effectively manage complex studies. In this context, CROs not only assist with the operational aspects of research studies but also play a strategic role in enhancing the overall quality and pace of drug development.
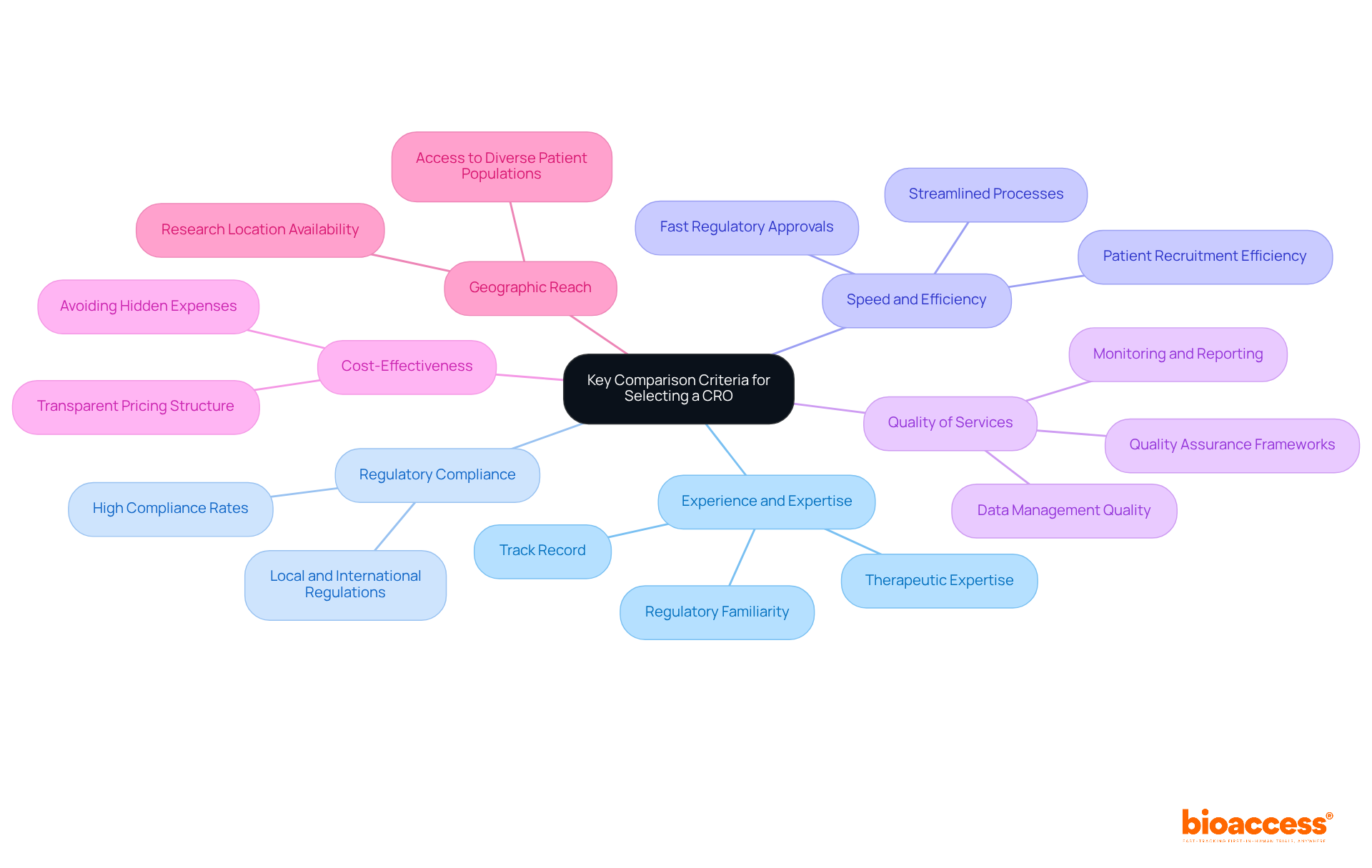
Choosing the appropriate clinical research organizations in Europe is crucial for the success of research studies. Understanding the essential criteria for selection is imperative for ensuring effective collaboration and successful outcomes.
Experience and Expertise: Assess the CRO's history in managing studies similar to yours, including their therapeutic expertise and familiarity with regulatory requirements. A strong track record indicates their capability to navigate complex challenges effectively.
Regulatory Compliance: Regulatory adherence is non-negotiable. Ensure the CRO adheres to both local and international regulations, as this is essential for preserving the integrity of clinical studies. High compliance rates among CROs are indicative of their commitment to quality and reliability.
Speed and Efficiency: Evaluate the CRO's ability to streamline processes, particularly in patient recruitment and regulatory approvals. For instance, bioaccess® offers regulatory approval in just 6-8 weeks, significantly faster than the typical 6-12 months in the US/EU, and can enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites. This capability can greatly impact study timelines and enhance overall project efficiency.
Quality of Services: Investigate the quality of data management, monitoring, and reporting services offered by the CRO. Quality Assurance frameworks are essential for ensuring transparency and reproducibility throughout the testing phases.
Cost-Effectiveness: Review the CRO's pricing structure to ensure it aligns with your project's budgetary constraints. Transparency in cost structures is crucial to avoid hidden expenses that could arise during the trial.
Geographic Reach: Consider the CRO's access to various patient populations and research locations. A wide geographic coverage can boost recruitment initiatives and enhance data diversity, which is becoming more crucial in today's research environment.
Integrating these standards into your selection procedure for clinical research organizations in Europe will assist in ensuring that you collaborate with an organization able to provide successful research outcomes. Furthermore, bioaccess® links cutting-edge Medtech and Biopharma startups with leading clinical research locations, further improving the potential for successful study execution.
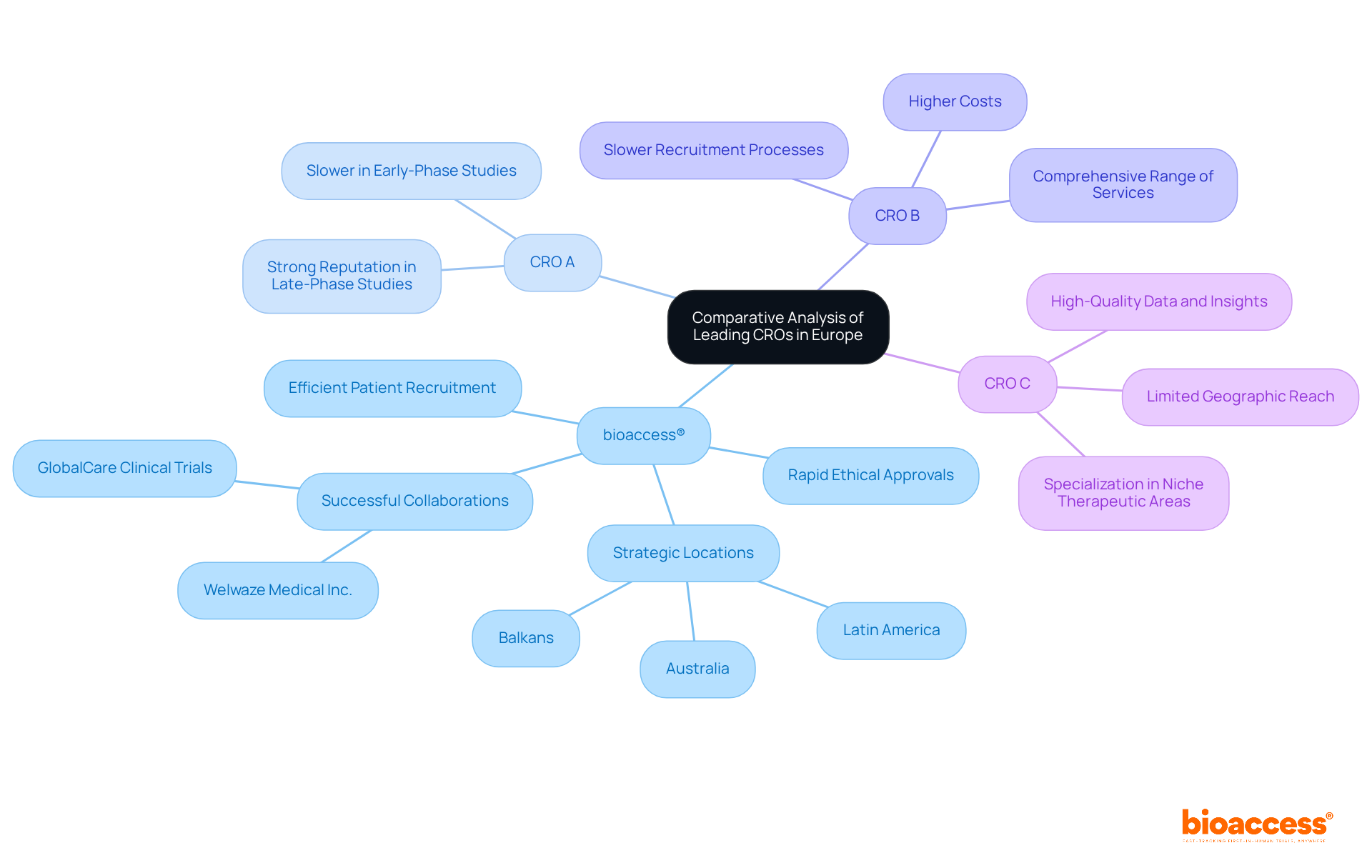
In Europe, several leading CROs distinguish themselves based on key performance metrics:
bioaccess®: Renowned for its rapid ethical approvals and efficient patient recruitment, bioaccess® capitalizes on its strategic locations in Latin America, the Balkans, and Australia. This positioning enables the organization to deliver ethical approvals in just 4-6 weeks and achieve enrollment rates that are 50% faster than traditional markets. Their specialization in early-phase studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Clinical Follow-Up Studies, not only accelerates timelines but also offers cost-effective solutions tailored to the needs of Medtech, Biopharma, and Radiopharma innovators. Notably, bioaccess® has successfully collaborated with Welwaze Medical Inc. for the launch of the Celbrea® medical device in Colombia, showcasing its regulatory and market access expertise. Furthermore, bioaccess®'s partnership with GlobalCare Clinical Trials has resulted in over a 50% reduction in recruitment time and a 95% retention rate, showcasing its effectiveness in patient recruitment and retention.
CRO A: This organization boasts a strong reputation for its extensive experience in late-phase studies. However, it often falls short in speed compared to bioaccess®, particularly in early-phase studies where rapid progression is critical.
CRO B: While offering a comprehensive range of services, CRO B is frequently criticized for its higher costs and slower recruitment processes. This can hinder the overall efficiency of medical trials, especially when rapid patient enrollment is essential.
CRO C: Specializing in niche therapeutic areas, CRO C provides high-quality data and insights. However, its limited geographic reach compared to bioaccess® may restrict patient diversity, which is increasingly important in medical research.
Overall, bioaccess® distinguishes itself in the competitive landscape of clinical research organizations in Europe, particularly due to its agility in early-phase research studies and its capability to effectively navigate complex regulatory environments. The increasing prevalence of chronic diseases in Europe further underscores the demand for bioaccess®'s services, as these conditions drive the need for innovative treatments.
Choosing the appropriate clinical research organizations in Europe is vital for the success of clinical studies, particularly in aligning their strengths with the specific requirements of the research. Here are key recommendations:
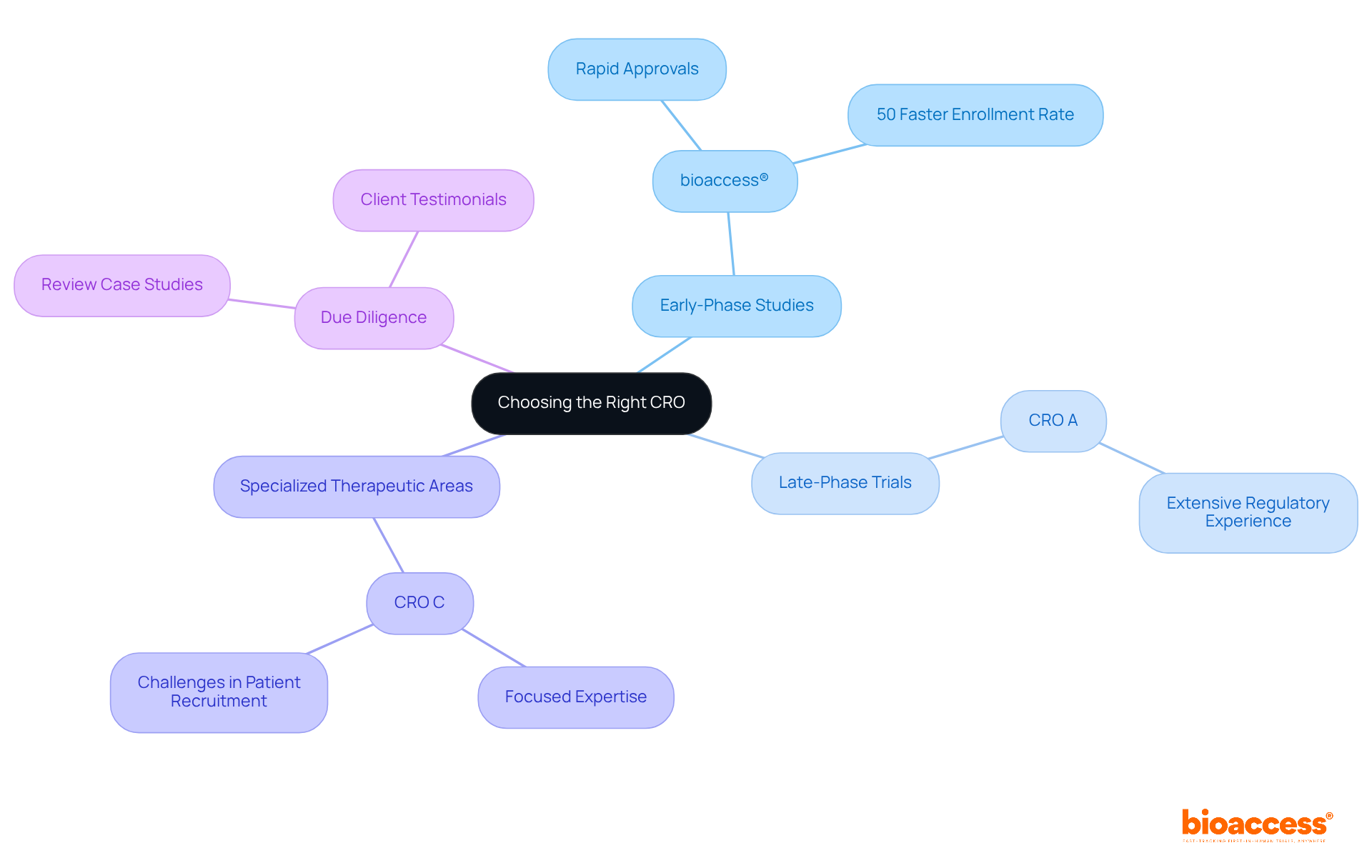
The pivotal role of clinical research organizations (CROs) in Europe is undeniably significant, serving as essential partners in the pharmaceutical and biotechnology sectors. By offering a comprehensive range of services—from study design to regulatory compliance—CROs enhance the efficiency and success of clinical trials. As the market for contract research outsourcing continues to expand, understanding how to select the right CRO becomes increasingly crucial for ensuring that innovative therapies reach the market promptly.
Key insights from this analysis underscore the importance of:
when choosing a CRO. Organizations such as bioaccess® distinguish themselves through their rapid ethical approvals and effective patient recruitment strategies, while others may excel in late-phase studies or specialized therapeutic areas. A comparative assessment of leading CROs in Europe reveals the diversity of their strengths, emphasizing the necessity for careful consideration of the specific requirements of each research project.
In a landscape where the demand for innovative treatments is surging, selecting the right CRO is imperative. Stakeholders are encouraged to conduct thorough due diligence, leveraging case studies and testimonials to make informed decisions. By aligning with the appropriate CRO, organizations can not only enhance their operational efficiency but also significantly contribute to the advancement of medical research and the development of new therapies that can improve patient outcomes.
What is the role of Clinical Research Organizations (CROs) in Europe?
CROs in Europe play a crucial role in the pharmaceutical, biotechnology, and medical device industries by offering outsourced research services that optimize the study process, including managing trials, ensuring regulatory compliance, and facilitating data collection and analysis.
What key services do CROs provide?
Key services provided by CROs include study design, site selection, patient recruitment, data management, regulatory submissions, and detailed reporting on study status and adverse events.
How do CROs impact research study efficiency?
CROs significantly enhance research study efficiency by improving patient recruitment strategies, which helps mitigate the risks associated with studies that fail to meet enrollment targets, potentially saving pharmaceutical firms from substantial financial losses.
What are the financial implications of failed medical studies?
Approximately 80% of medical studies fail to meet initial enrollment targets, leading to significant financial losses estimated at $8 million daily for pharmaceutical development firms.
How are collaborations between CROs and pharmaceutical firms viewed in the industry?
Collaborations between CROs and pharmaceutical firms are recognized as vital for achieving trial success, with effective cooperation yielding better results and faster timelines.
What is the projected growth of the contract research outsourcing market?
The global market for contract research outsourcing is projected to reach $90.4 billion by 2030, reflecting a compound annual growth rate of 7.7%.
What strategic role do CROs play in drug development?
CROs assist with the operational aspects of research studies and play a strategic role in enhancing the overall quality and pace of drug development, which is increasingly important as pharmaceutical and biotech firms rely on them to manage complex studies.