

This article presents a comprehensive comparison of different antibody types, underscoring their unique functions and applications within the immune response and medical fields. It elaborates on the distinct roles of each antibody type—IgG, IgM, IgA, IgD, and IgE—in both immunity and therapeutic contexts. This emphasis on their relevance in diagnostics, treatment strategies, and vaccine development illustrates the critical importance of understanding these differences to achieve optimal clinical outcomes.
Antibodies stand as the unsung heroes of the immune system, intricately designed to identify and neutralize foreign invaders. With five distinct types—IgG, IgM, IgA, IgD, and IgE—each plays a specialized role in immune defense and therapeutic applications. As the medical field continues to evolve, understanding the unique functions and applications of these immunoglobulins becomes increasingly vital.
How do these differences influence their effectiveness in treating diseases?
What implications do they hold for future medical advancements?
These questions underscore the critical importance of antibodies in the landscape of clinical research.
Antibodies, also known as immunoglobulins, represent specialized Y-shaped glycoproteins synthesized by B-cells in response to antigens. Each immune protein comprises four polypeptide chains: two identical heavy chains and two identical light chains. The variable regions of these chains create specific antigen-binding sites, enabling immune proteins to recognize and bind to distinct antigens. Conversely, the constant region defines the class of immunoglobulin—such as IgG, IgA, IgM, IgD, and IgE—and dictates its role in the immune response, including the activation of complement proteins and interaction with different antibody types and various immune cells.
Recent studies underscore the significance of immunoglobulin structure in modulating immune responses, revealing that the pentameric configuration of IgM provides ten antigen-binding sites, thereby enhancing its avidity and effectiveness in defending against early infections. Furthermore, advancements in immunoglobulin research have led to innovative applications, including the deployment of monoclonal proteins in targeted therapies for conditions such as cancer and autoimmune diseases. Understanding the intricate relationship between immune protein structure and function is essential for leveraging these glycoproteins in clinical settings and improving therapeutic strategies.
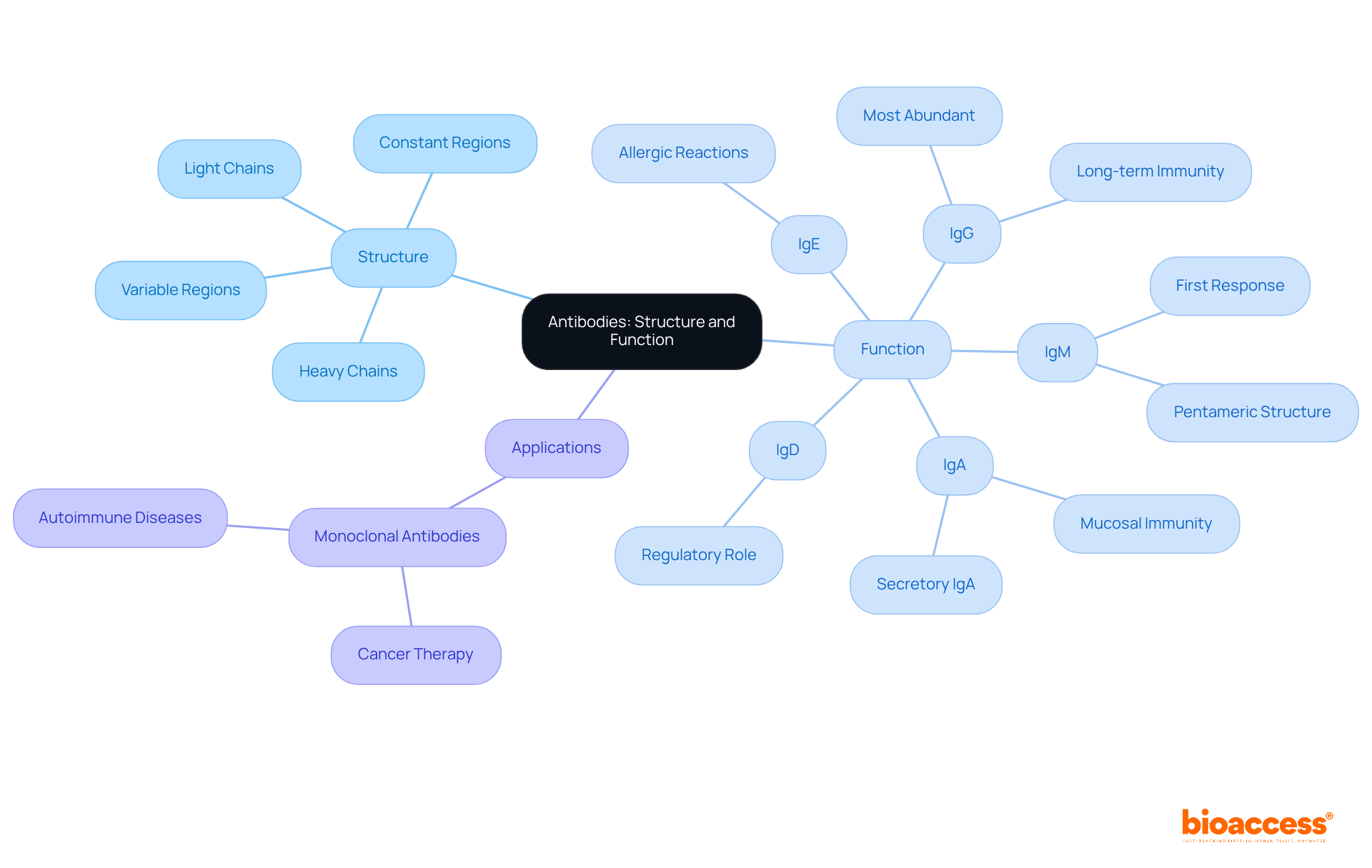
The five primary types of antibodies are classified according to their heavy chain structure and function, each playing a crucial role in the immune response:
The immune system comprises different antibody types, each with distinct functions that make them suitable for various therapeutic applications.
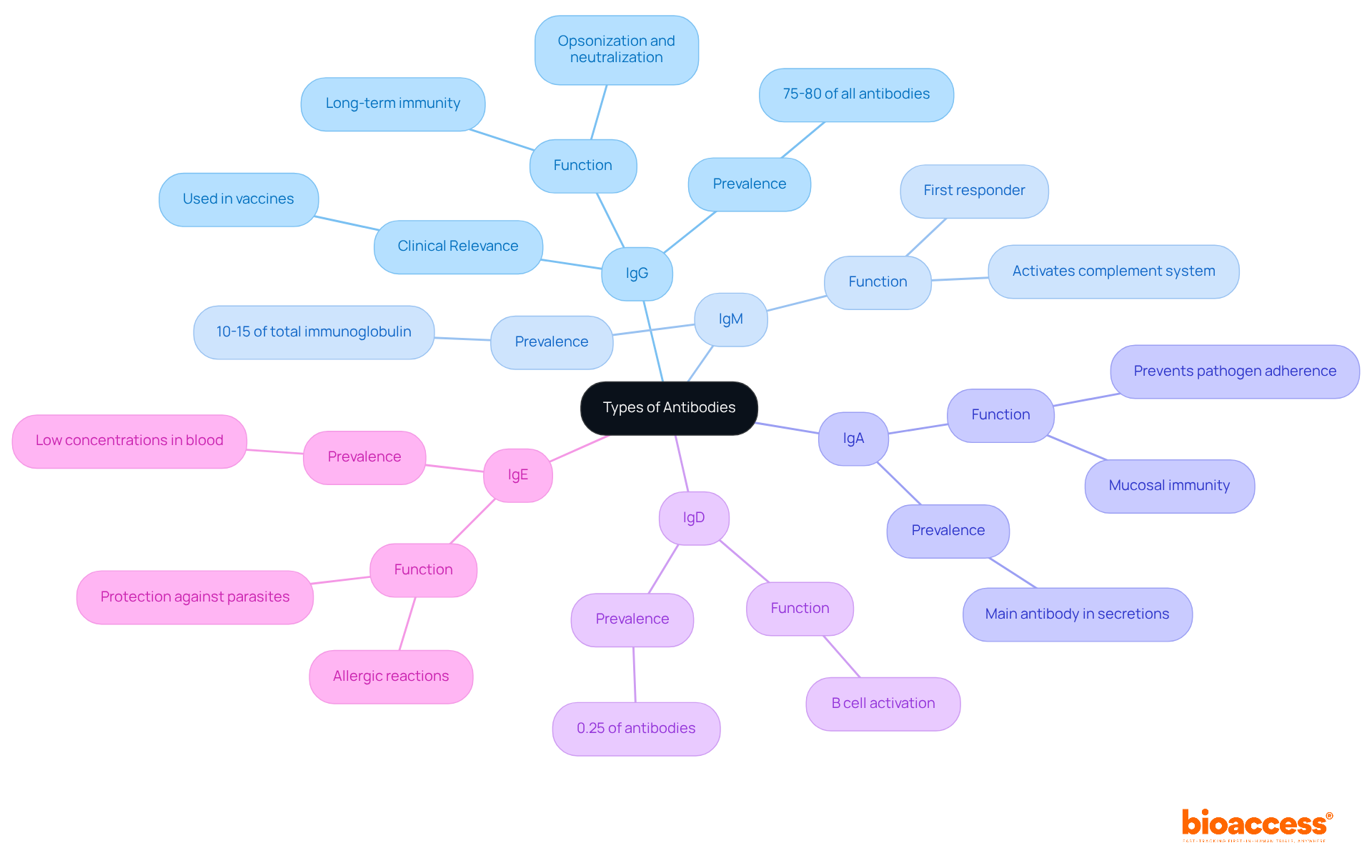
Antibodies play a crucial role in medicine and research, with each type serving distinct functions:
IgG: Predominantly used in therapeutic monoclonal antibodies, IgG is essential for treating diseases such as cancer and autoimmune disorders. Its ability to cross the placenta is vital for providing passive immunity to newborns.
IgM: This immune protein is pivotal in diagnostic tests for acute infections, as it is the first immunoglobulin produced in response to an infection. Its pentameric structure enhances its capacity to agglutinate pathogens, making it a reliable marker for early detection. Recent studies indicate that combining IgM and IgG detection significantly increases positivity rates, achieving 72.73% for negative nucleic acid tests and 87.50% for positive tests. This underscores IgM's critical role in improving diagnostic accuracy for infections.
IgA: Important for vaccine development, particularly mucosal vaccines, IgA provides localized immunity in the respiratory and gastrointestinal tracts, enhancing protection against pathogens at entry points.
IgD: Although less understood, IgD is crucial for B cell activation, which is essential for developing targeted therapies that enhance immune responses.
IgE: Targeted in allergy therapies, anti-IgE monoclonal proteins are effective in managing allergic conditions such as asthma and chronic urticaria.
The varied uses of these proteins emphasize their importance in progressing medical science and enhancing patient results, especially in the realm of infectious disease diagnostics and therapeutic measures.

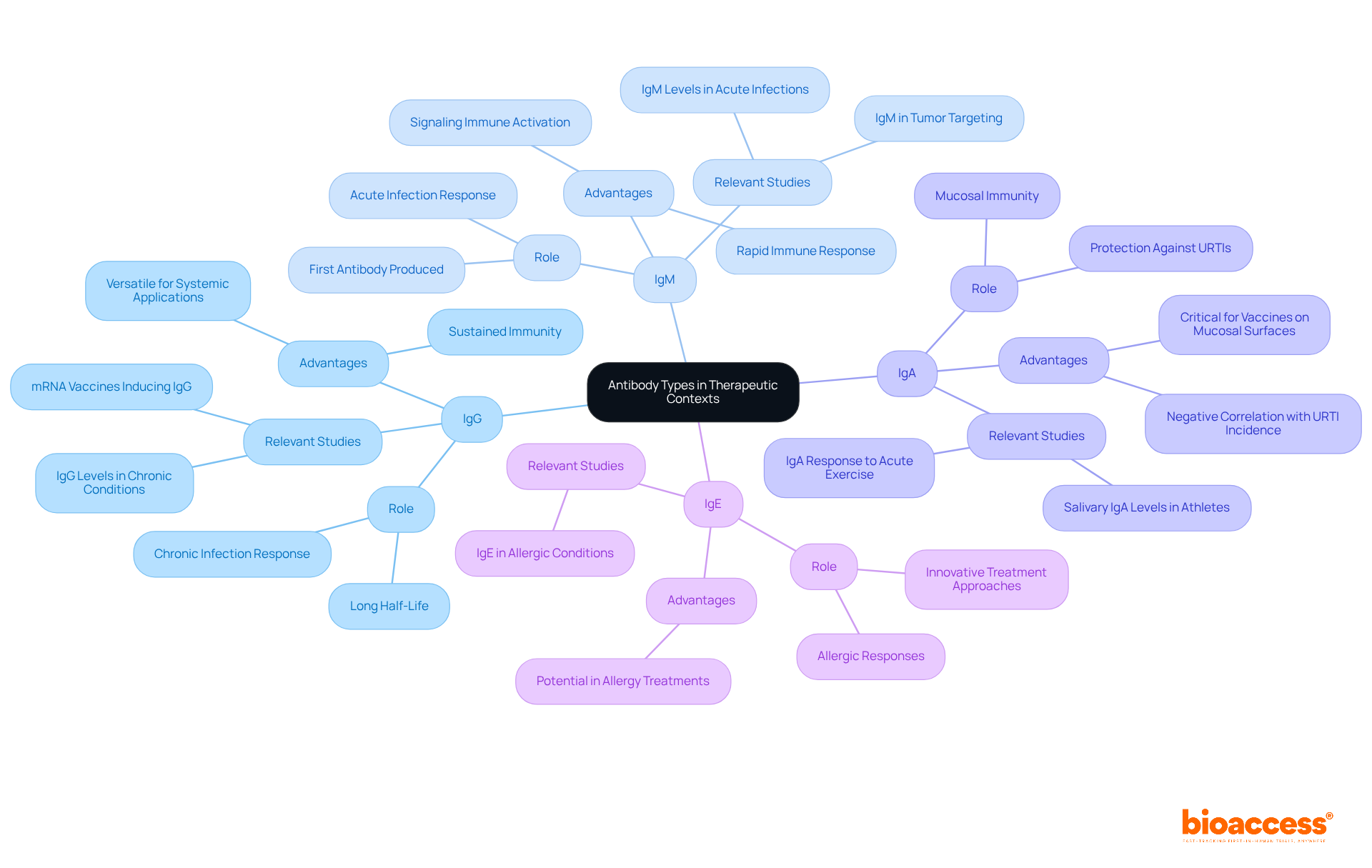
In therapeutic contexts, understanding the distinctions between antibody types is paramount for achieving optimal patient outcomes.
IgG vs. IgM: IgG is the preferred choice for chronic conditions, attributed to its long half-life and capacity to maintain sustained immunity. Conversely, IgM excels in acute infections, providing a rapid immune response. Notably, IgM is the first immunoglobulin produced during an infection, effectively signaling the immune system to activate additional defense mechanisms.
IgA vs. IgG: IgA plays a critical role in mucosal immunity, making it particularly suitable for vaccines aimed at mucosal surfaces. In contrast, IgG demonstrates greater versatility for systemic applications. Recent studies underscore that mRNA vaccines have elicited the highest levels of spike-specific IgG and IgA proteins, emphasizing the importance of both in vaccine efficacy. Furthermore, a significant negative correlation has been identified between the incidence of upper respiratory tract infections (URTI) and IgA levels, reinforcing IgA's protective function against infections.
IgE: Although not commonly utilized in standard therapies, IgE's involvement in allergic responses has inspired innovative treatment approaches aimed at mitigating allergic conditions.
Recognizing these differences is crucial for selecting the appropriate antibody type tailored to specific therapeutic objectives. This understanding ensures that clinical research can effectively address patient needs and enhance treatment outcomes.
Understanding the diverse types of antibodies and their specific functions is essential for appreciating their crucial roles in both the immune system and medical applications. Each antibody type—IgG, IgM, IgA, IgD, and IgE—serves distinct purposes, from providing long-term immunity to facilitating immediate responses during infections, thereby forming a comprehensive defense mechanism against various pathogens.
The article highlights the unique characteristics and applications of each antibody type, emphasizing their significance in therapeutic contexts.
By recognizing these differences, healthcare professionals can tailor therapies to meet specific patient needs, improving outcomes in areas such as cancer treatment and infectious disease diagnostics.
Ultimately, the exploration of antibody types and their functionalities underscores the importance of ongoing research in immunology and therapeutic development. As the understanding of these immune proteins expands, so too does the potential for innovative treatments that can enhance patient care and tackle complex health challenges. Embracing this knowledge not only enriches the field of medicine but also empowers individuals to make informed decisions about their health and well-being.
What are antibodies and their primary function?
Antibodies, also known as immunoglobulins, are specialized Y-shaped glycoproteins synthesized by B-cells in response to antigens. Their primary function is to recognize and bind to distinct antigens, aiding in the immune response.
What is the structure of antibodies?
Antibodies are composed of four polypeptide chains: two identical heavy chains and two identical light chains. The variable regions of these chains create specific antigen-binding sites, while the constant region defines the class of immunoglobulin.
What are the different classes of immunoglobulins?
The classes of immunoglobulins include IgG, IgA, IgM, IgD, and IgE. Each class has distinct roles in the immune response.
How does the structure of IgM enhance its function?
The pentameric configuration of IgM provides ten antigen-binding sites, which enhances its avidity and effectiveness in defending against early infections.
What are some recent advancements in immunoglobulin research?
Recent advancements include the development of monoclonal proteins for targeted therapies in conditions such as cancer and autoimmune diseases.
Why is understanding the structure and function of antibodies important?
Understanding the intricate relationship between antibody structure and function is essential for leveraging these glycoproteins in clinical settings and improving therapeutic strategies.