


The key differences between control and treatment groups are fundamental to clinical research, with the control group not receiving the experimental intervention while the treatment group does. This distinction is crucial for establishing causality and minimizing bias, as it enables researchers to accurately assess the intervention's effects against a standard or placebo benchmark. Understanding these roles is essential for ensuring the integrity and validity of clinical trials, thereby enhancing the overall reliability of research outcomes.
In the realm of clinical research, the distinction between control and treatment groups serves as a cornerstone for validating the effectiveness of new interventions. By carefully analyzing these groups, researchers can discern whether observed effects stem from the experimental treatment or other variables.
However, the ethical and logistical challenges of managing these groups often raise critical questions:
Delving into the dynamics of control and treatment groups reveals not only their significance in clinical trials but also the complexities that underpin their implementation.
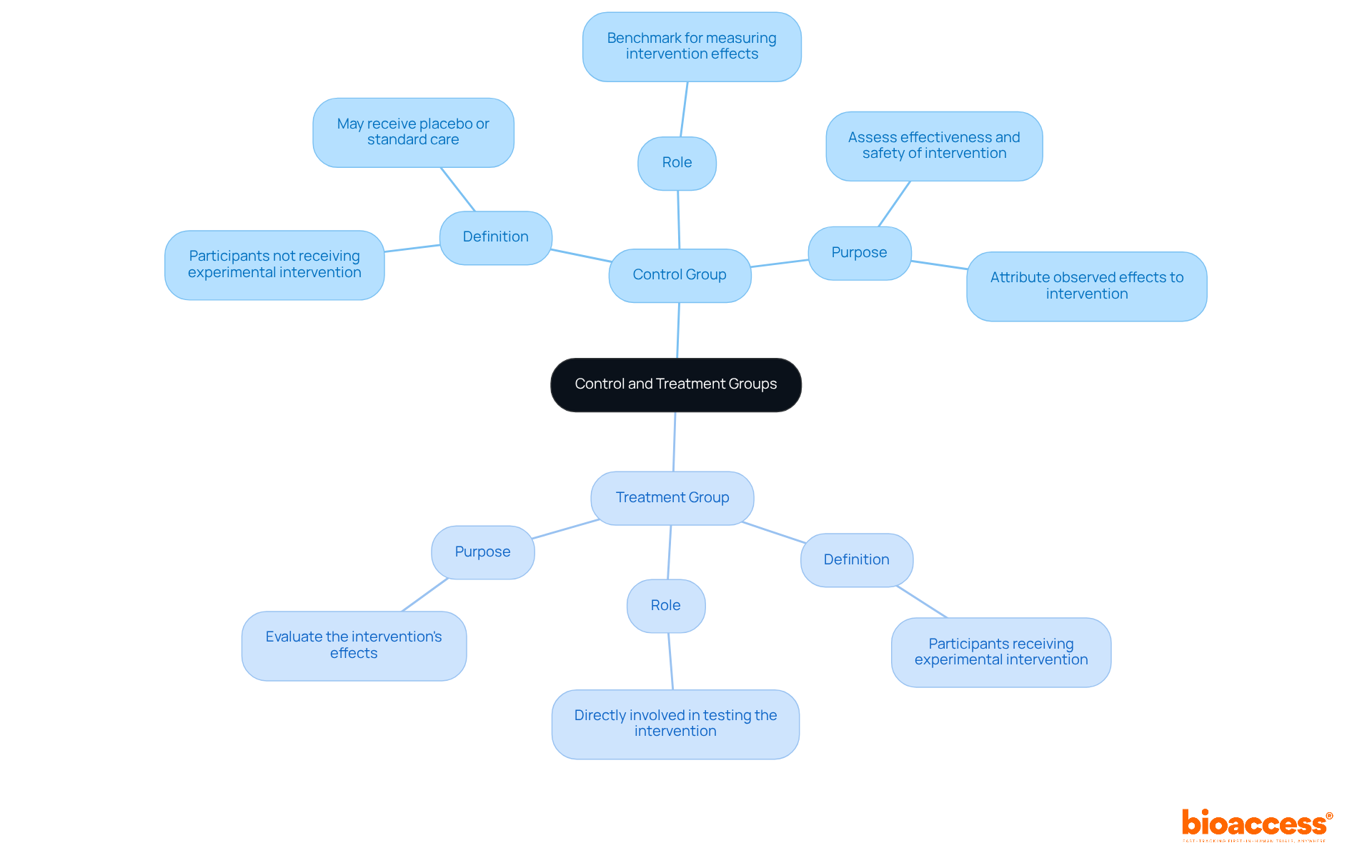
In clinical research, the control group treatment group is made up of participants who do not receive the experimental procedure or intervention. Instead, they may receive a placebo or standard care, serving as a benchmark against which the effects of the intervention can be measured. Conversely, a subject cohort comprises participants who receive the experimental intervention being tested.
The primary objective of comparing the control group and treatment group is to assess the effectiveness and safety of the intervention by analyzing the results between the two sets. This distinction is crucial for ensuring that any observed effects can be attributed to the intervention itself rather than extraneous factors.
At bioaccess®, our 20+ years of experience in overseeing trials, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), empowers us to efficiently design and monitor the control group treatment group, ensuring robust and reliable outcomes in the research process.
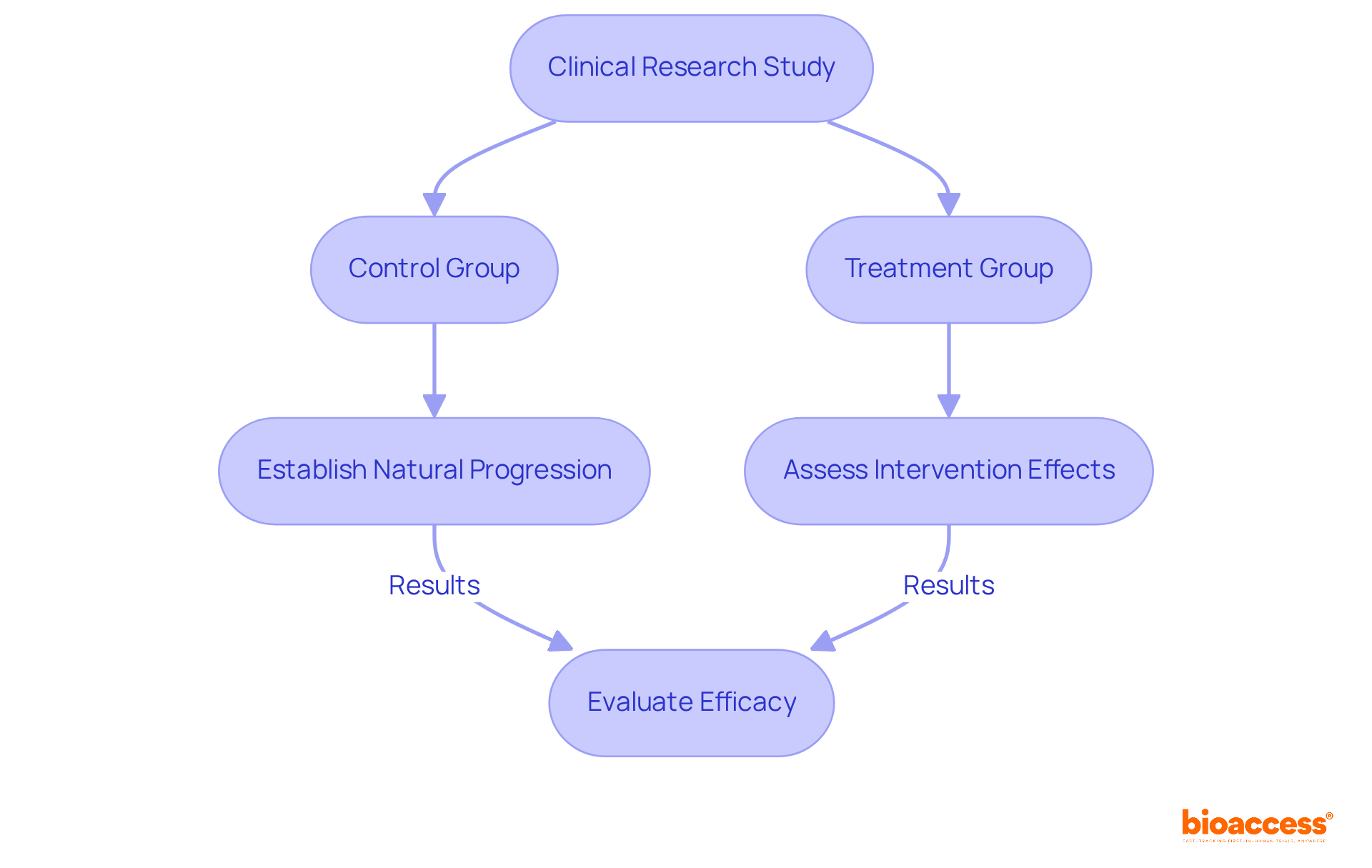
In clinical research, the control group and treatment group cohorts are pivotal, as they establish a causal relationship between the intervention and the observed outcomes. For instance, in ReGelTec's Early Feasibility Study on HYDRAFIL™ for chronic low back pain, the meticulous arrangement of the control group and treatment group sections is crucial for assessing the efficacy of this innovative hydrogel solution.
By comparing results from the control group and the treatment group, researchers can determine whether HYDRAFIL™ significantly affects patients with degenerative disc disease. This comparison is essential for minimizing bias and ensuring that results remain unaffected by external factors.
Furthermore, control sets facilitate the recognition of a condition's natural progression, allowing researchers to distinguish between the intervention's effects and the disease's natural course. Such methodological rigor is vital for regulatory approval and for fostering trust among stakeholders, including patients and healthcare providers.
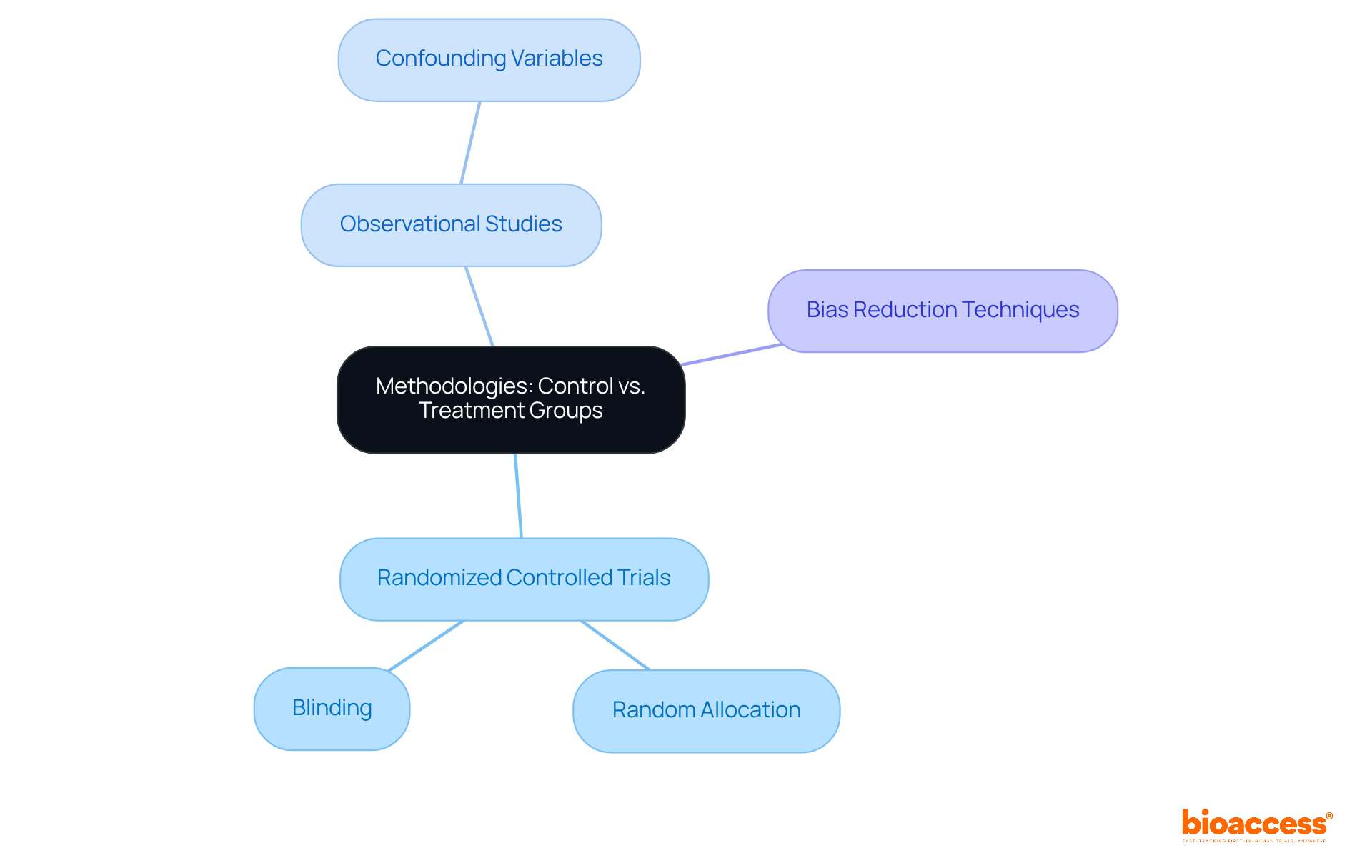
The approaches for management and intervention categories can differ greatly based on the research design. In randomized controlled trials (RCTs), participants are randomly allocated to either the control group or treatment group, effectively eliminating selection bias and ensuring comparability between the groups. Blinding, where participants and/or researchers are unaware of group assignments, is often employed to further reduce bias. In contrast, observational studies may not utilize randomization, which can introduce potential confounding variables that affect the results. Understanding these methodologies is essential for analyzing the results of medical trials and evaluating the reliability of the findings.
At bioaccess®, we leverage over 20 years of expertise in overseeing trials, including:
Our customized strategy for the control group and treatment group is supported by thorough compliance evaluations and efficient project oversight, enhancing the reliability of trial results and establishing us as a trusted partner in navigating the complexities of clinical research.
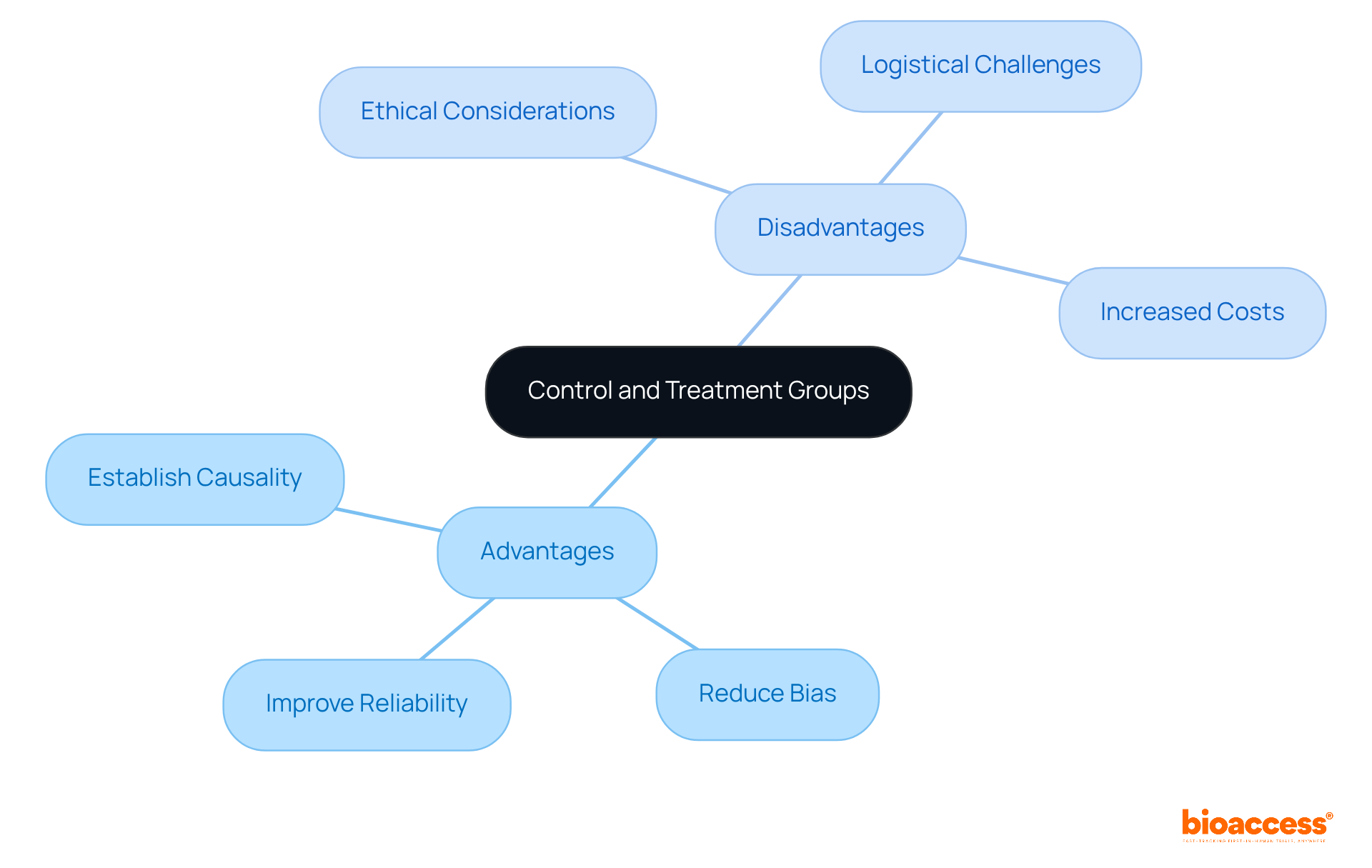
The benefits of utilizing a control group treatment group alongside experimental sets are significant, as they enable researchers to establish causality, reduce bias, and improve the reliability of outcomes. Control group treatment group sets provide a standard for comparison, while experimental sets allow for an examination of the intervention's effects.
However, challenges arise, particularly ethical considerations when withholding treatment from the control group treatment group in studies involving serious conditions. Furthermore, the complexity of managing these groups can lead to logistical challenges and increased costs.
At bioaccess®, we understand these challenges and offer extensive trial management services, including:
Our expertise in conducting Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) ensures that we can effectively balance these factors, designing clinical trials that yield meaningful results.
Understanding the distinctions between control and treatment groups is essential for conducting effective clinical research. These groups play a pivotal role in establishing the causal relationships necessary to evaluate the effectiveness of new interventions. A well-structured control group serves as a benchmark, enabling researchers to discern the true impact of the treatment group, thus ensuring that the observed effects are attributable to the intervention rather than external variables.
Throughout this article, key aspects such as the methodologies employed in randomized controlled trials versus observational studies have been explored. The importance of minimizing bias through randomization and blinding has been emphasized, alongside the ethical considerations and logistical challenges that arise when managing these groups. The insights provided illustrate how a meticulous approach to trial design can significantly enhance the reliability of research outcomes.
In light of these insights, it is crucial for researchers and stakeholders in the clinical field to fully appreciate the significance of control and treatment groups. By leveraging robust methodologies and addressing the inherent challenges, the potential for meaningful advancements in medical research is greatly enhanced. Engaging with these principles not only fosters trust among participants and healthcare providers but also drives the pursuit of innovative solutions that can transform patient care.
What are control and treatment groups in clinical research?
In clinical research, the control group consists of participants who do not receive the experimental intervention, often receiving a placebo or standard care instead. The treatment group, or subject cohort, includes participants who receive the experimental intervention being tested.
What is the primary objective of comparing control and treatment groups?
The primary objective is to assess the effectiveness and safety of the intervention by analyzing the results between the two groups, ensuring that any observed effects can be attributed to the intervention itself rather than extraneous factors.
How does bioaccess® contribute to the management of control and treatment groups?
Bioaccess® has over 20 years of experience in overseeing trials, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), which enables them to efficiently design and monitor the control and treatment groups, ensuring robust and reliable outcomes in the research process.