


The article centers on the development of an effective clinical research coordinator (CRC) description, emphasizing the essential responsibilities, qualifications, and integration of company culture. A well-crafted CRC description should articulate core tasks, including:
Furthermore, it underscores the significance of qualifications, such as:
All framed within the organization's values and culture. This approach is crucial for attracting the right candidates who align with the company’s mission.
Crafting an effective job description for a Clinical Research Coordinator (CRC) is essential for attracting the right talent to manage the complexities of clinical trials. This article explores the fundamental responsibilities and qualifications of the role, emphasizing the importance of aligning it with company culture. Such alignment not only enhances recruitment efforts but also ensures that candidates resonate with the organization's values.
However, how can organizations ensure that their job descriptions not only outline the necessary skills and duties but also connect with potential candidates' values and aspirations?
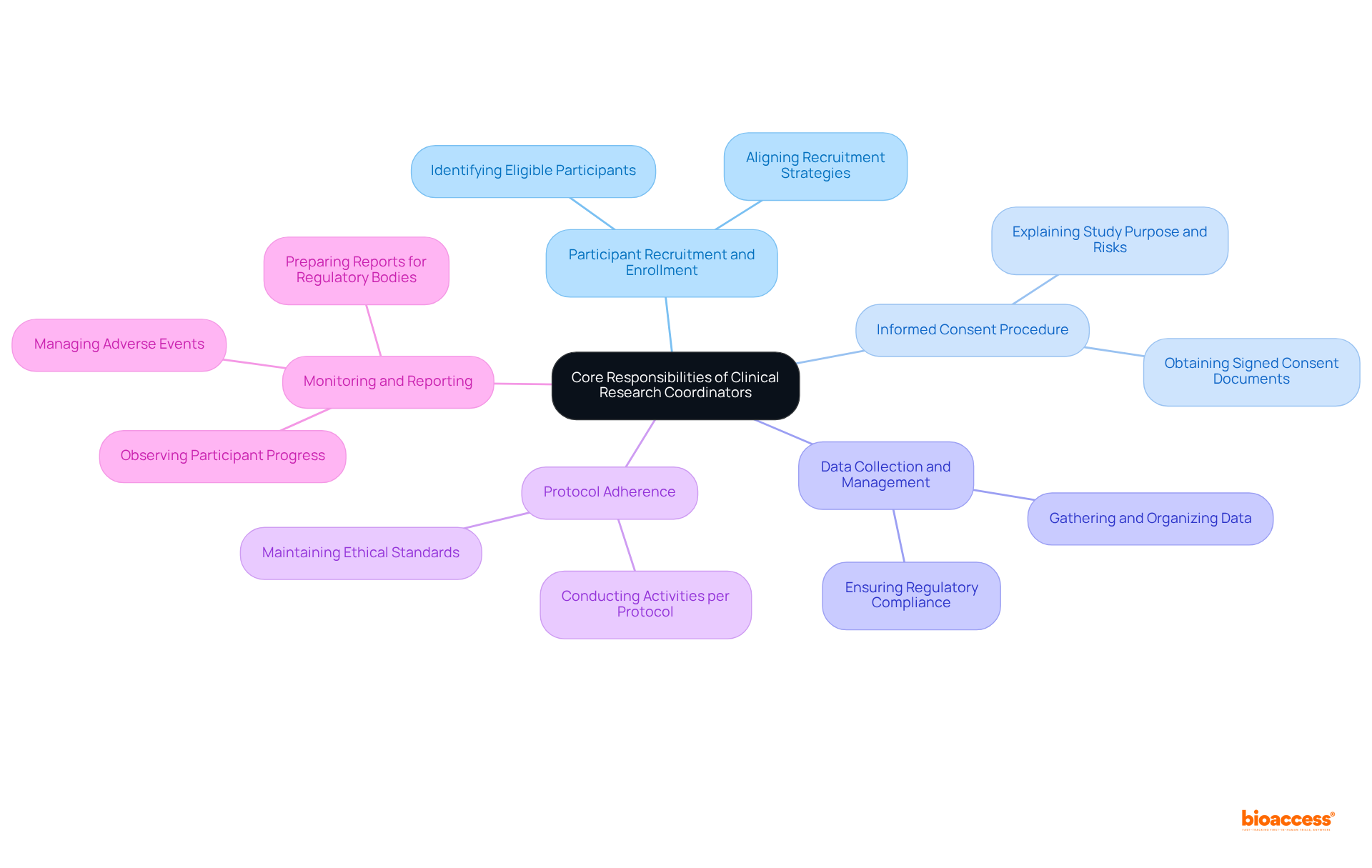
The clinical research coordinator description highlights their essential role in the management of clinical trials. The clinical research coordinator description highlights that their core responsibilities are pivotal in ensuring the success of clinical research initiatives.
Participant Recruitment and Enrollment: CRCs are tasked with identifying and enrolling eligible participants, ensuring that recruitment strategies are meticulously aligned with the study's objectives.
Informed Consent Procedure: They assist in the informed consent procedure, ensuring that participants fully comprehend the purpose, procedures, risks, and benefits associated with the study.
Data Collection and Management: The clinical research coordinator description includes the responsibilities of gathering, organizing, and preserving precise data throughout the research process, ensuring strict adherence to regulatory standards and protocols.
Protocol Adherence: They ensure that all study activities are conducted in accordance with the study protocol, regulatory requirements, and ethical guidelines, thereby maintaining the integrity of the research.
The clinical research coordinator description indicates that CRCs serve as the primary point of contact for participants, investigators, and sponsors, facilitating effective communication and collaboration among all parties involved.
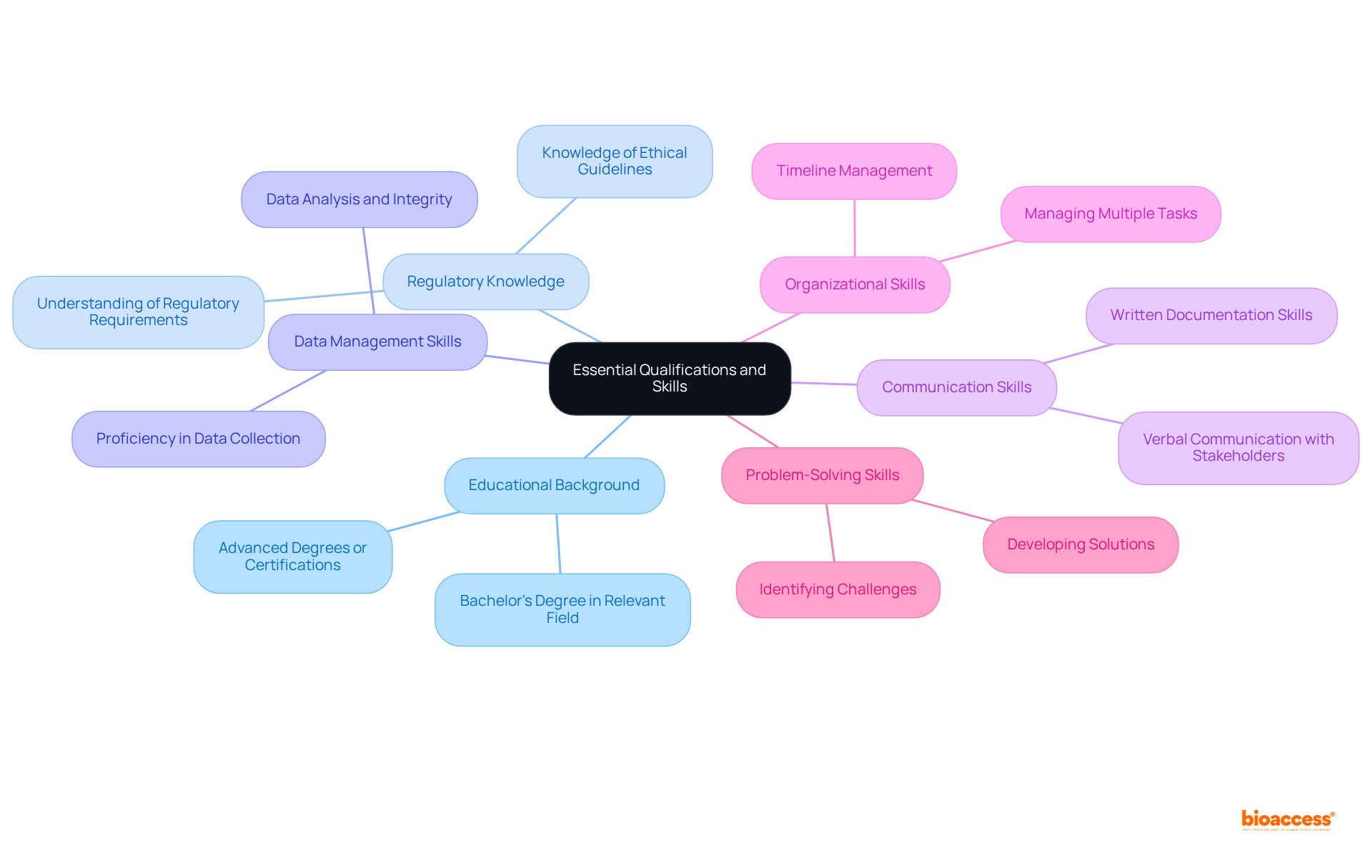
To excel in the role, candidates must possess the essential qualifications and skills outlined in the clinical research coordinator description.
Educational Background: A bachelor's degree in a relevant field such as life sciences, nursing, or public health is typically required. Having advanced degrees or certifications in clinical studies can significantly enhance a candidate's profile as outlined in the clinical research coordinator description.
Regulatory Knowledge: A solid grasp of regulatory requirements and ethical guidelines governing clinical studies is crucial for a clinical research coordinator description to ensure compliance. This knowledge ensures that all research activities align with established standards.
Data Management Skills: Proficiency in data collection, management, and analysis is vital for preserving the integrity of research data, as outlined in the clinical research coordinator description. Effective data management safeguards the accuracy and reliability of study outcomes.
The clinical research coordinator description emphasizes that excellent verbal and written communication skills are necessary for effective interaction with participants, investigators, and regulatory bodies. Clear communication fosters collaboration and understanding among all stakeholders.
Organizational Skills: The clinical research coordinator description highlights that strong organizational abilities are required to manage multiple tasks, timelines, and stakeholders effectively. This skill set is essential for navigating the complexities of clinical trials.
Problem-Solving Skills: The clinical research coordinator description highlights that clinical researchers must be adept at identifying and addressing challenges that arise during the study process. This capability ensures that investigations remain on track and objectives are met.
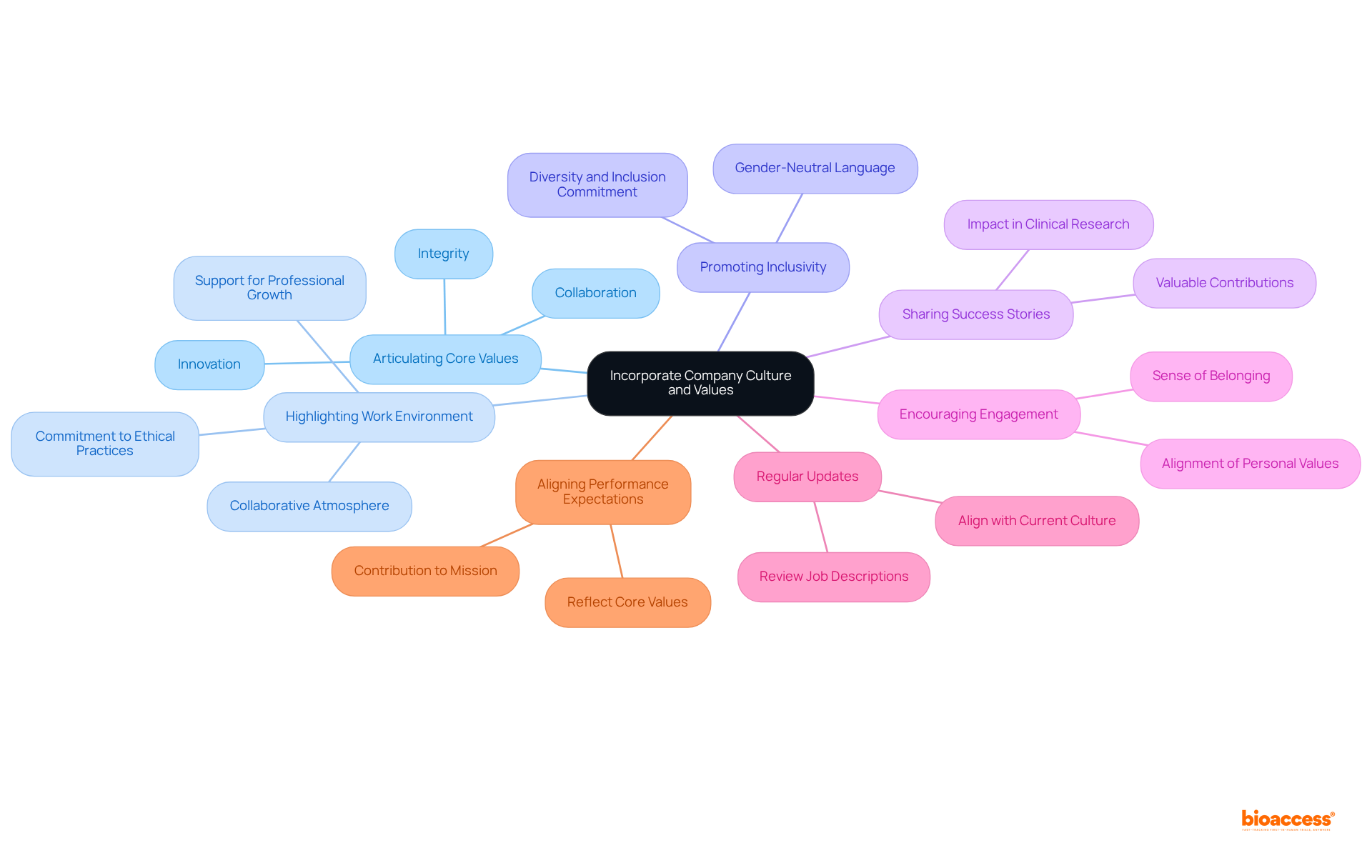
In crafting a clinical research coordinator description, embedding the company's culture and values is crucial. This can be effectively achieved through several key strategies:
By integrating these elements, organizations can create a clinical research coordinator description that attracts qualified candidates while also reflecting a strong organizational culture, ultimately enhancing employee performance and satisfaction in the clinical research sector.
Crafting a successful clinical research coordinator description is essential for attracting the right talent and ensuring the effectiveness of clinical trials. Clearly defining the core responsibilities, qualifications, and organizational culture enables companies to create a compelling narrative that resonates with potential candidates, ultimately enhancing the recruitment process.
Outlining specific responsibilities such as:
is crucial, as is identifying the essential skills needed for success in this role. Moreover, highlighting the organization's values and culture not only draws in qualified candidates but also fosters a sense of belonging and purpose, which is vital for employee satisfaction and retention.
In the competitive field of clinical research, a well-crafted job description serves as a vital tool for aligning candidates with the organization's mission and values. Organizations are encouraged to regularly update these descriptions to reflect evolving standards and expectations, ensuring relevance and effectiveness in attracting top talent. By prioritizing a comprehensive approach to job descriptions, companies can significantly enhance their recruitment efforts and contribute to the success of clinical research initiatives.
What are the core responsibilities of clinical research coordinators (CRCs)?
The core responsibilities of CRCs include participant recruitment and enrollment, assisting in the informed consent procedure, data collection and management, ensuring protocol adherence, serving as a primary point of contact, and monitoring and reporting on participant progress.
How do clinical research coordinators assist with participant recruitment?
CRCs identify and enroll eligible participants, ensuring that recruitment strategies align with the study's objectives.
What role do CRCs play in the informed consent procedure?
They assist in the informed consent procedure by ensuring that participants fully understand the purpose, procedures, risks, and benefits associated with the study.
What is involved in data collection and management for CRCs?
CRCs are responsible for gathering, organizing, and preserving accurate data throughout the research process, ensuring compliance with regulatory standards and protocols.
How do CRCs ensure protocol adherence?
They ensure that all study activities are conducted according to the study protocol, regulatory requirements, and ethical guidelines, maintaining the integrity of the research.
What is the communication role of clinical research coordinators?
CRCs serve as the primary point of contact for participants, investigators, and sponsors, facilitating effective communication and collaboration among all parties involved.
What monitoring and reporting responsibilities do CRCs have?
They monitor participant progress, manage adverse events, and prepare comprehensive reports for regulatory bodies and sponsors, ensuring transparency and accountability in the study process.