

Creating a compliant medical device list is essential for navigating the complexities of regulatory requirements. This process involves not only understanding the legal standards set by regulatory bodies such as the FDA and EMA but also accurately classifying devices and compiling the necessary documentation. Establishing a robust review process for ongoing compliance is crucial.
By following these steps, organizations can ensure adherence to legal standards, which ultimately facilitates the successful development and marketing of medical products.
Collaboration and strategic planning are vital in this landscape, as they address key challenges in clinical research and enhance the overall effectiveness of medical product development.
Navigating the intricate landscape of medical device compliance presents significant challenges, particularly due to the constantly evolving regulatory frameworks governing this industry. For manufacturers aiming to successfully introduce their products to market, a thorough understanding of the requirements established by authorities such as the FDA and EMA is imperative. This article delineates a clear, four-step approach to developing a compliant medical device list, offering valuable insights into regulatory classifications, documentation practices, and ongoing compliance strategies. Given the frequency of updates and the rigor of standards, how can manufacturers proactively ensure they stay ahead of the curve and avert costly pitfalls in their compliance journey?
Developing a compliant medical device list begins with a thorough understanding of the legal framework, particularly the standards established by the FDA in the United States and the EMA in Europe. This process involves several essential steps to ensure compliance:
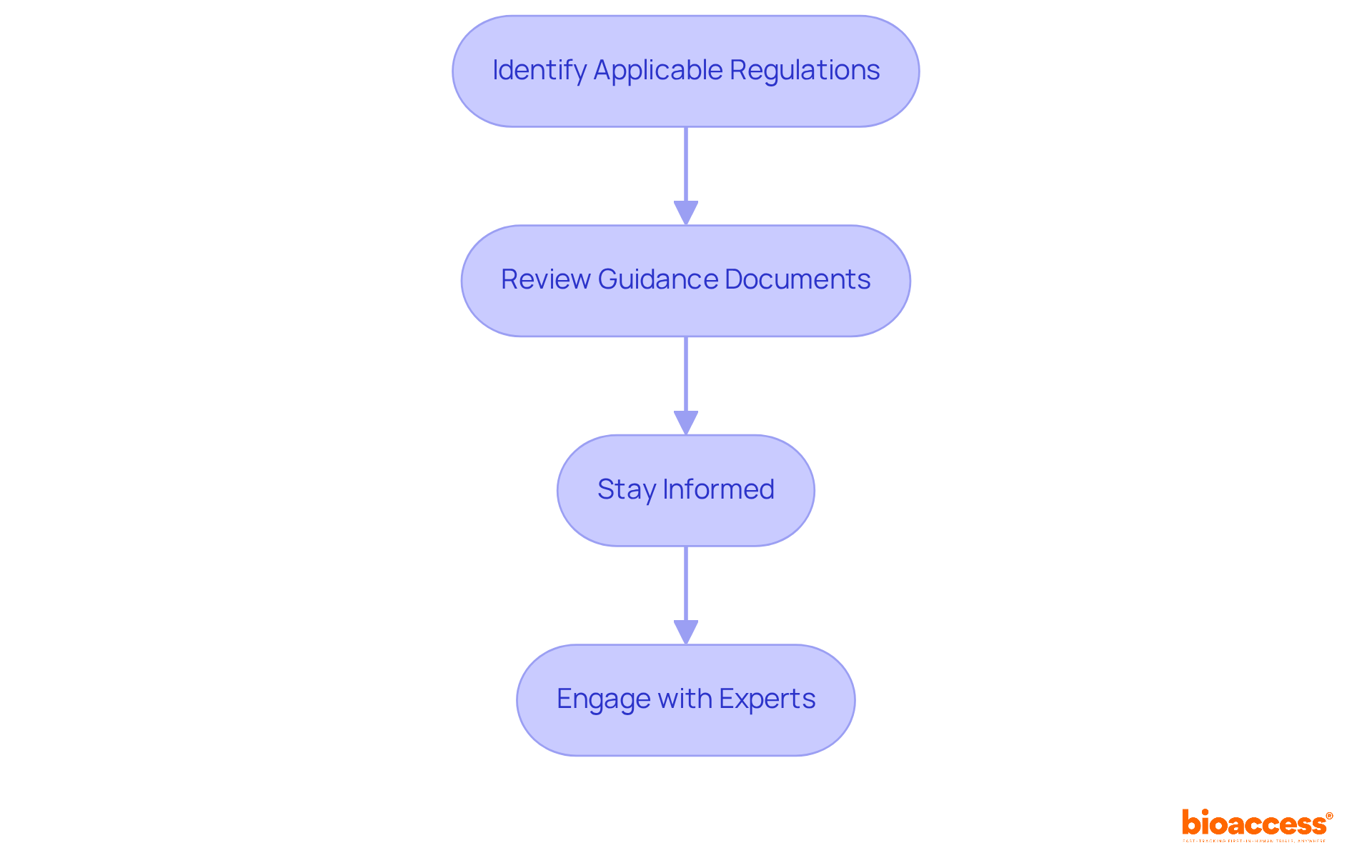
Identify Applicable Regulations: Different classes of devices—Class I, II, and III—are subject to varying regulatory requirements. Class I items, typically low-risk, may be exempt from premarket notification, while Class II items necessitate a 510(k) submission that demonstrates substantial equivalence to a predicate item. Class III products, however, require a more rigorous Premarket Approval (PMA) process, which demands extensive clinical data.
Review Guidance Documents: Regulatory agencies offer detailed guidance documents that outline the necessary adherence steps, including labeling requirements, safety protocols, and efficacy standards. Familiarizing yourself with these documents is critical for aligning your device with regulatory expectations.
Stay Informed: Regulatory frameworks are dynamic, with frequent updates that can impact compliance. Notably, nearly 32% of FDA 510(k) submissions did not pass the initial acceptance assessment in the year prior to September 2022. This statistic underscores the importance of staying updated on evolving standards, especially in light of the challenges posed by the new Medical Device Regulations (MDR) in the EU.
Engage with Experts: Collaborating with compliance professionals, such as Ana Criado, Director of Compliance at bioaccess, can significantly deepen your understanding of compliance requirements. Ana's extensive background, which includes her roles at Colombia’s oversight agency INVIMA and her expertise in biomedical engineering and health economics, positions her as an invaluable resource. As Aaron S. Kesselheim noted, "To navigate complexities and avoid common pitfalls, obtaining expert compliance support is essential for a smoother approval process." By leveraging the services provided by bioaccess, including trial set-up, project management, and reporting, you can facilitate the successful development and commercialization of your medical product.
By adhering to these steps, you can effectively navigate the compliance landscape, paving the way for the successful development and marketing of your medical product.
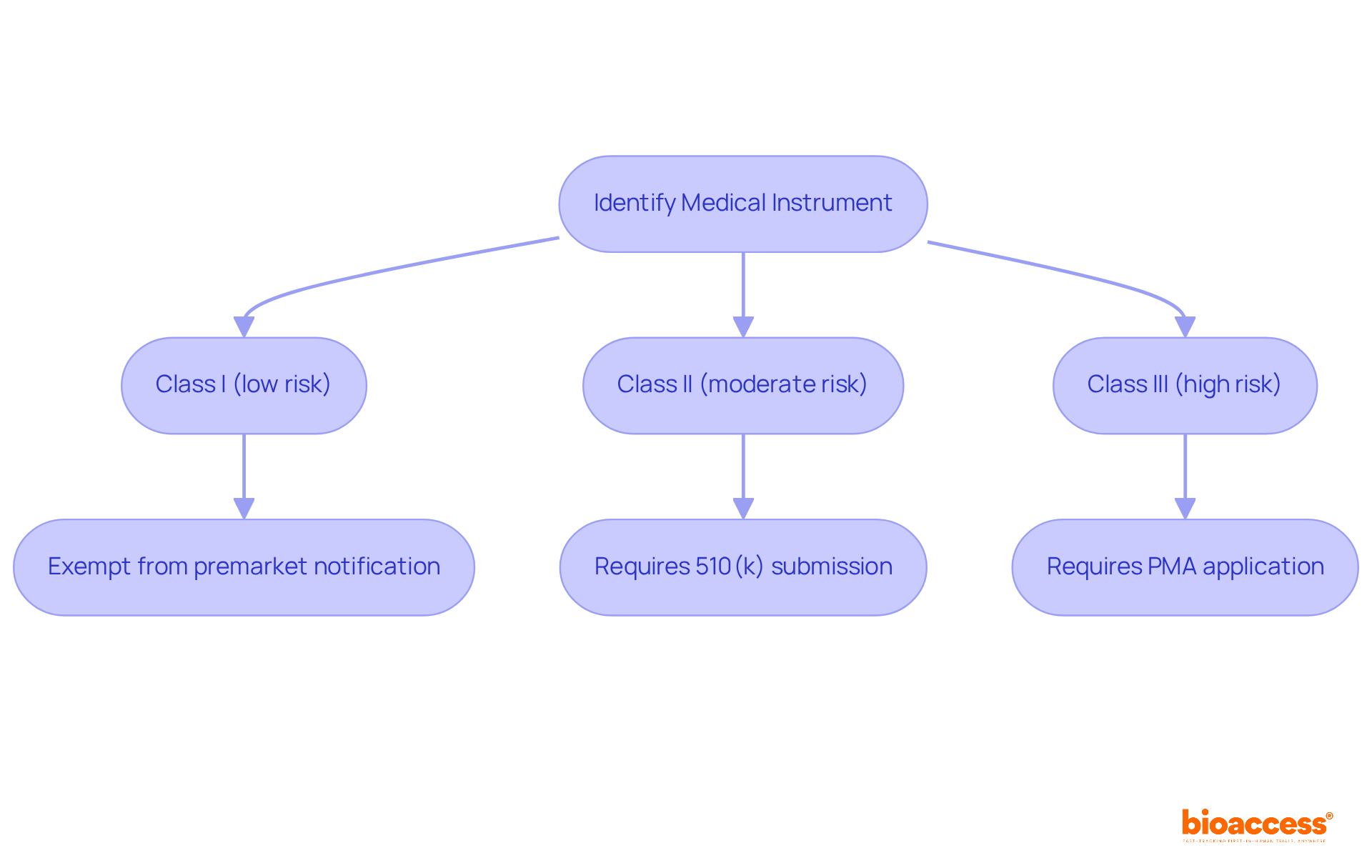
Next, categorize your medical instrument according to the medical device list and established guidelines. This involves understanding classification levels that are crucial for compliance with the medical device list in the Medtech landscape. According to the medical device list, medical instruments are classified into three categories:
Each class has specific regulatory requirements; Class I items are typically exempt from premarket notification, while Class II items necessitate a 510(k) submission demonstrating substantial equivalence to a product on the medical device list. Notably, Class III products, which account for approximately 10% of all medical equipment, are part of the medical device list and are subject to the most rigorous regulations, requiring a premarket approval (PMA) application.
Consult classification resources to ensure accurate categorization. Utilize resources such as the FDA’s classification database, which provides essential information on the medical device list, regulation numbers, and classifications. Recent updates in 2025 reflect evolving frameworks, particularly for AI-enabled tools and digital health technologies. This emphasizes the necessity for manufacturers to stay informed about changes in the medical device list to remain compliant and competitive.
Identify applicable standards relevant to your equipment after classification. This may include ISO standards, such as ISO 13485 for quality management systems, or specific performance standards pertinent to your device type. Understanding and adhering to the medical device list standards is crucial for compliance and market access, which reinforces your position within the industry.
Finally, document classification decisions meticulously. Maintain detailed records of your classification process and the rationale behind your decisions. This documentation is essential for compliance submissions and can help mitigate risks linked to improper classification, which can lead to costly delays and reclassification requests. Engaging with the FDA early in the process can provide clarity and prevent missteps, ensuring a smoother pathway to market.
Following classification, it is crucial to compile all pertinent information regarding your medical device, focusing on the following key components:
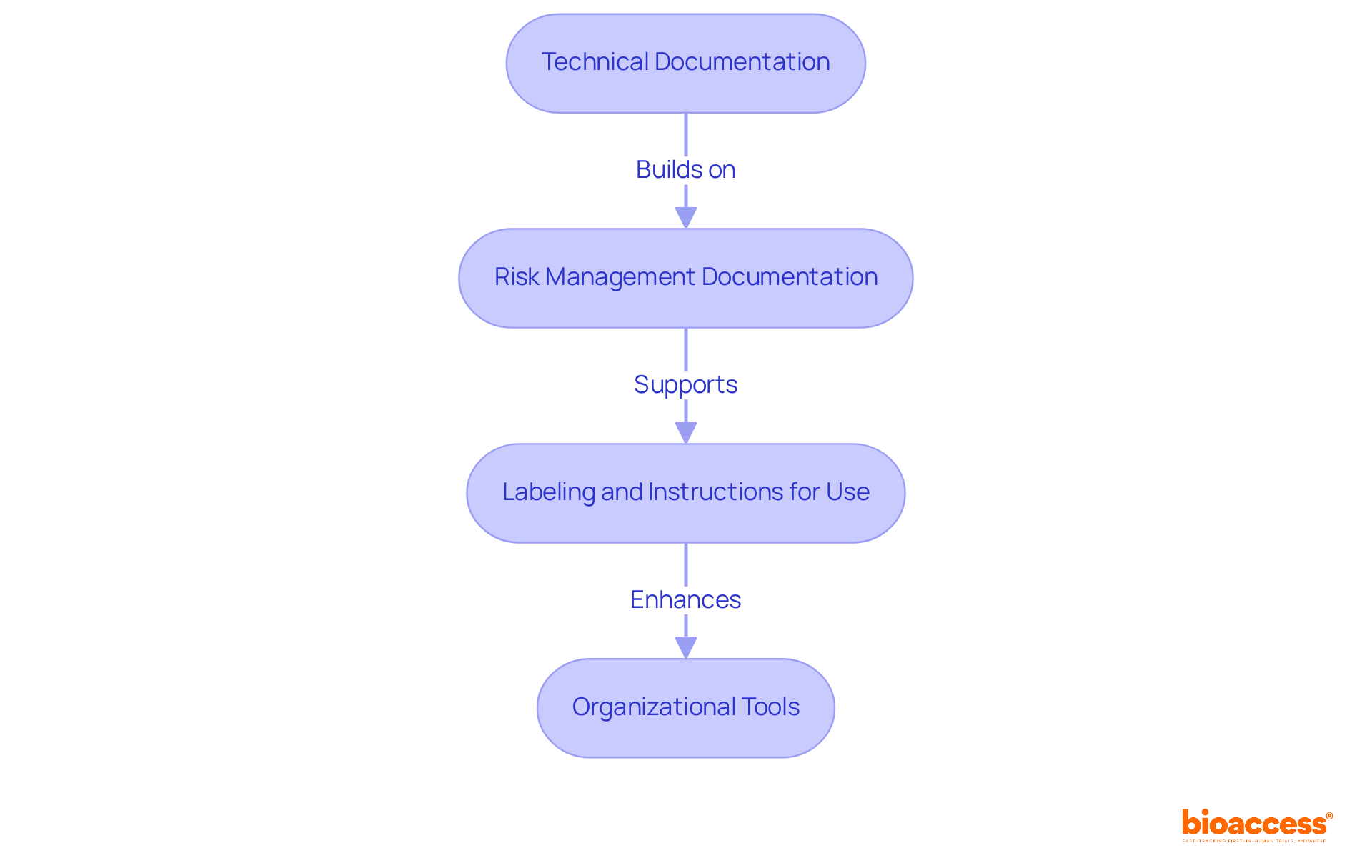
Technical Documentation: Develop a comprehensive technical file that encompasses design specifications, manufacturing processes, and performance data. This documentation serves as a foundation for regulatory compliance and product safety.
Risk Management Documentation: Comply with ISO 14971 by documenting risk assessments and management plans. This standard underscores a systematic approach to identifying and mitigating risks throughout the device's lifecycle, ensuring that all potential hazards are proactively addressed.
Labeling and Instructions for Use: Produce labeling and user instructions that meet legal standards. These materials should be clear, informative, and designed to enhance user understanding, thereby minimizing the likelihood of misuse.
Organizational Tools: Implement document management systems or software to systematically organize and store all documentation. This approach facilitates straightforward access to information, optimizing regulatory processes and audits.
By focusing on these aspects, manufacturers can ensure that their documentation not only meets the medical device list standards but also supports effective risk management practices, ultimately enhancing product safety and market readiness.
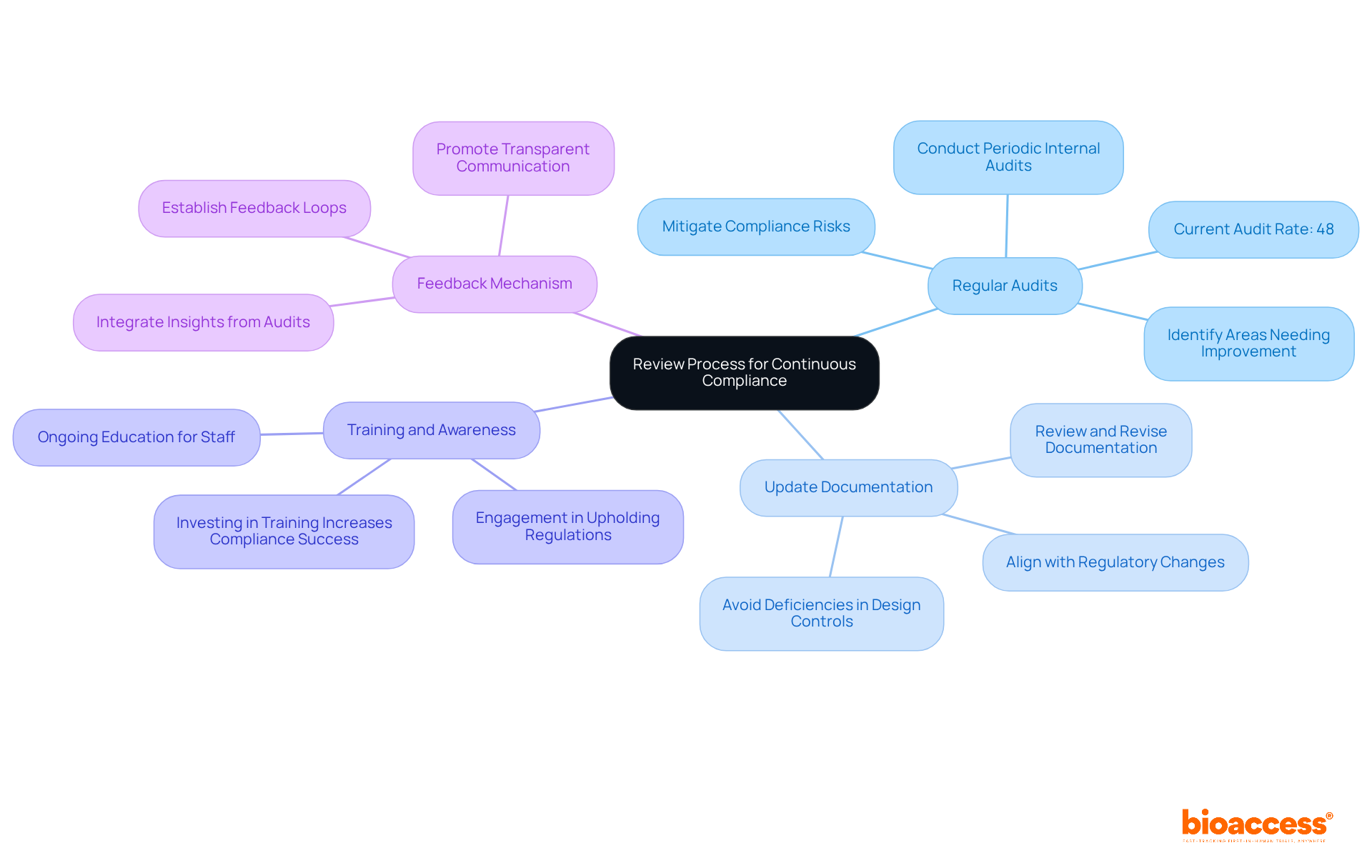
To guarantee continuous adherence to established standards, implementing a robust review procedure is crucial. This process should encompass several key components:
Regular Audits: Conduct periodic internal audits to evaluate compliance with regulatory requirements. These audits help identify areas needing improvement and ensure effective execution of regulatory measures. Notably, organizations that routinely audit high-risk areas are better positioned to mitigate compliance risks; only 48% of healthcare organizations currently perform such audits. This statistic underscores the need for enhanced audit practices, especially given the significant rise in Warning Letters issued to medical manufacturers, as highlighted in the medical device list, increasing from 24 in the previous year to 47 in fiscal year 2024.
Update Documentation: Consistently review and revise all documentation to align with any changes in regulations or the medical device list. This practice is vital, particularly in light of the rising number of Warning Letters highlighting deficiencies such as design controls and CAPA issues. Meticulous documentation practices are essential to avoid similar pitfalls.
Training and Awareness: Provide ongoing education for your team regarding regulatory requirements and best practices. This ensures that all personnel are well-informed and actively engaged in upholding regulations. Entities investing in training are more likely to navigate legal challenges successfully, as continuous education fosters a culture of adherence.
Feedback Mechanism: Establish a feedback loop to gather insights from audits and team members. This mechanism facilitates ongoing improvement in regulatory processes, ensuring that insights gained from audits are integrated into future practices. By promoting a culture of transparent communication, organizations can enhance their adherence strategies and adapt to evolving legal frameworks.
In addition to these elements, leveraging comprehensive clinical trial management services, such as those offered by bioaccess, can significantly bolster your adherence efforts. Services including feasibility studies, site selection, compliance reviews, trial setup, import permits, and project management are essential for navigating the complexities of legal requirements. With the expertise of professionals like Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics in Colombia, organizations can ensure a thorough and compliant approach to clinical research.
Creating a compliant medical device list is a multifaceted process that demands a profound understanding of regulatory requirements, careful classification, meticulous documentation, and ongoing compliance reviews. By adhering to the outlined steps, manufacturers can adeptly navigate the complexities of medical device regulations, ensuring their products satisfy the necessary standards for market entry.
Key insights emphasize the critical need to:
Regular audits and updates to documentation are paramount for continuous compliance, alongside fostering a culture of training and feedback within organizations. These practices not only mitigate risks but also elevate the overall quality and readiness of medical devices for commercialization.
Ultimately, the significance of a compliant medical device list cannot be overstated. It serves as a foundational element for ensuring patient safety, promoting innovation, and maintaining a competitive advantage in the Medtech landscape. Manufacturers are urged to prioritize compliance as an ongoing commitment, leveraging expert resources and adopting best practices to effectively navigate the ever-evolving regulatory environment.
What are the main regulatory bodies for medical devices in the United States and Europe?
The main regulatory bodies are the FDA (Food and Drug Administration) in the United States and the EMA (European Medicines Agency) in Europe.
What are the different classes of medical devices and their regulatory requirements?
Medical devices are classified into three classes: Class I (typically low-risk, may be exempt from premarket notification), Class II (requires a 510(k) submission demonstrating substantial equivalence), and Class III (requires a rigorous Premarket Approval (PMA) process with extensive clinical data).
Why is it important to review guidance documents from regulatory agencies?
Reviewing guidance documents is critical as they outline necessary adherence steps, including labeling requirements, safety protocols, and efficacy standards to align your device with regulatory expectations.
How often do regulatory frameworks for medical devices change?
Regulatory frameworks are dynamic and frequently updated, which can impact compliance.
What percentage of FDA 510(k) submissions did not pass the initial acceptance assessment in the year prior to September 2022?
Nearly 32% of FDA 510(k) submissions did not pass the initial acceptance assessment.
Why is it beneficial to engage with compliance experts?
Collaborating with compliance professionals can deepen your understanding of requirements and help navigate complexities, ultimately facilitating a smoother approval process.
What services does bioaccess provide to support compliance in medical device development?
Bioaccess offers services including trial set-up, project management, and reporting to assist in the successful development and commercialization of medical products.