


Creating a Clinical Evaluation Report (CER) is a vital step in navigating the complex landscape of medical device regulations, particularly within the framework of the European Union Medical Device Regulation (EU MDR). This article delves into the essential requirements and best practices for developing a comprehensive CER, highlighting the importance of demonstrating device safety and performance through rigorous clinical evaluation. As manufacturers strive for compliance and successful market entry, understanding the stages of clinical evaluation, the significance of robust data management, and the utilization of templates and checklists becomes crucial.
By addressing common challenges and offering strategic solutions, this article aims to equip stakeholders with the knowledge needed to enhance the quality and integrity of their CERs, ultimately contributing to the advancement of medical technology and patient safety.
Creating a comprehensive Clinical Evaluation Report (CER) demands a thorough understanding of the European Union Medical Device Regulation (EU MDR) requirements. The EU MDR creates a rigorous structure for assessments, emphasizing the need for producers to prove the safety and efficacy of their medical instruments effectively. Central to this process is the medical assessment, which acts as a thorough appraisal of a product's safety and performance based on medical data, either obtained directly from the producer or collected independently.
To align with EU MDR, manufacturers must familiarize themselves with specific articles and annexes that outline the evaluation process. This includes recognizing the intended use of the apparatus and understanding the state of the art within the relevant therapeutic or diagnostic area, which is crucial for identifying minimum performance and safety expectations.
An effective CER is not merely a regulatory requirement but a vital component of the technical documentation necessary for CE marking in the European market. 'It is essential to compile a list of eligibility criteria for clinical trials, determining both inclusion and exclusion parameters that ensure participant safety and effectiveness of the apparatus.'. This structured approach helps to streamline the evaluation process, thereby minimizing potential risks and enhancing compliance with regulatory standards.
As emphasized by industry authorities, "In the dynamic environment of medical equipment regulations, ensuring the safety and efficacy of healthcare products is of utmost importance." By adhering to the regulatory framework and leveraging insights from experienced consultants, manufacturers can navigate the complexities of the EU MDR efficiently, paving the way for successful product certification and market entry.

The assessment process for medical devices is organized into several crucial phases: strategic planning, data gathering, data analysis, and reporting. Initiating this process begins with the creation of a comprehensive Clinical Evaluation Plan (CEP). This document sets the foundation by detailing objectives, scope, and methodologies, ensuring that all stakeholders are aligned and that the evaluation meets regulatory requirements.
Data collection proceeds, where strong medical evidence is gathered. This includes literature reviews, clinical investigations, and importantly, post-market monitoring information. The latter is often underutilized but is critical in providing insights into the device's ongoing safety and efficacy in real-world settings. As noted by industry experts, rigorously analyzing this information alongside primary information is crucial for an accurate assessment.
Next, the gathered information undergoes meticulous analysis. 'This stage is paramount; a common challenge observed is the lack of detailed analysis and coherent presentation of the information.'. It’s essential to ensure that tables and references clearly narrate the information story, preventing disconnections between conclusions and the information presented. 'Discrepancies frequently emerge among different documents like the evaluation plan, assessment report, and risk management files, emphasizing the necessity for a unified method to information gathering and analysis strategies.'.
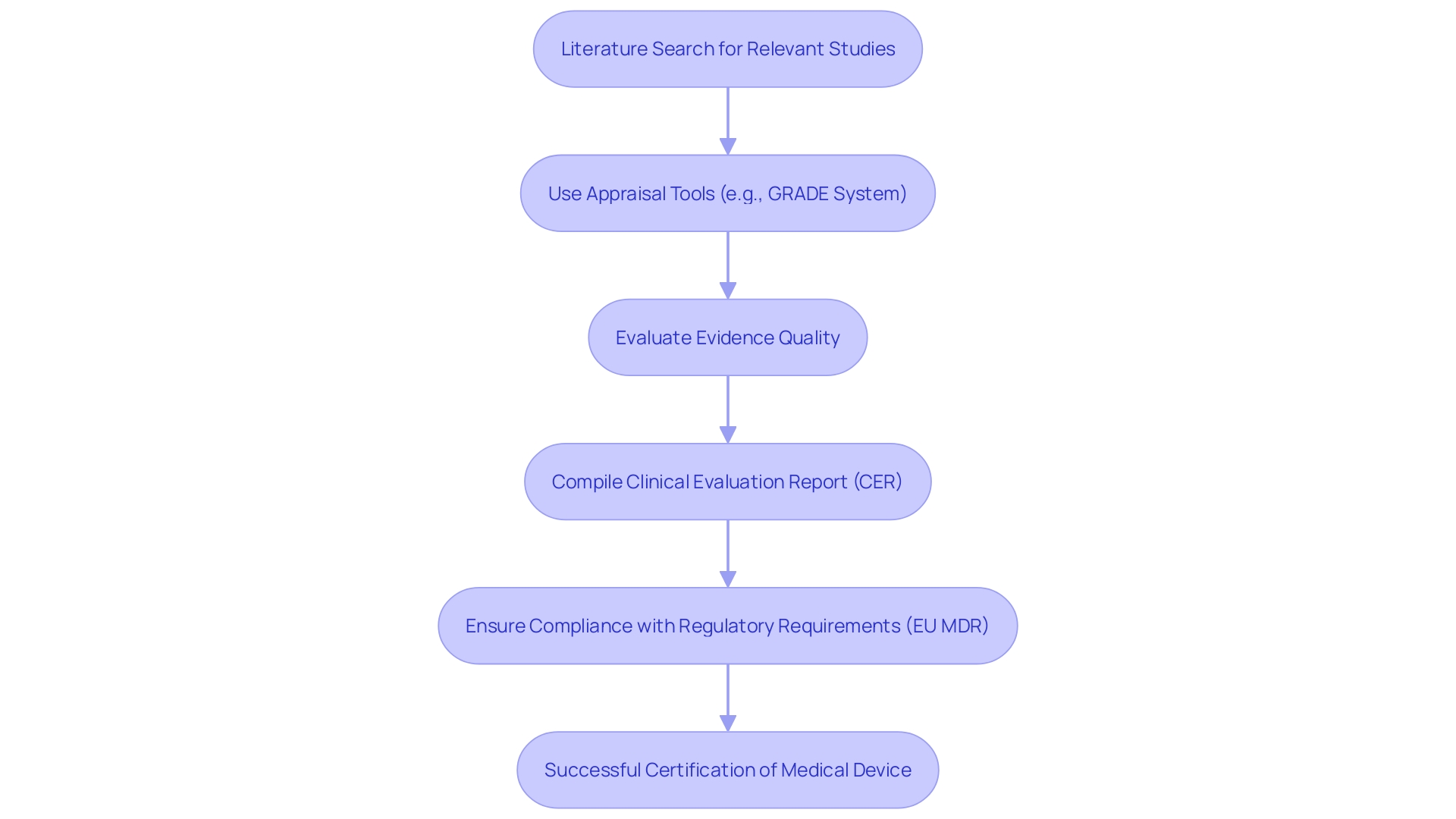
Finally, the findings are compiled into a Clinical Evaluation Report (CER). A well-constructed CER is not only a regulatory requirement but serves as a pivotal component in demonstrating the safety and performance of the product. The report must encapsulate all gathered evidence effectively, ensuring transparency and clarity in communication. This thorough documentation is crucial for securing CE marking in the European Union, ultimately facilitating market access and long-term adoption.

A comprehensive Clinical Evaluation Report (CER) hinges on the meticulous identification and appraisal of clinical information. Initiating a thorough literature search is paramount; it should focus on studies that are directly relevant to the medical device in question and its intended application. This search must encompass a wide array of information sources to ensure that the material retrieved is both comprehensive and representative.
Utilizing appraisal tools such as the GRADE system can significantly enhance the evaluation process. The GRADE framework allows researchers to assess the quality of evidence by considering factors such as study design, risk of bias, inconsistency, indirectness, imprecision, and publication bias. By applying this systematic methodology, researchers can ascertain the reliability and relevance of the information in supporting claims of safety and effectiveness.
In accordance with Article 62 of the European Union Medical Device Regulation (EU MDR), research studies must be structured to safeguard the rights and welfare of participants while ensuring that the information produced is scientifically valid and reliable. This regulatory structure highlights the significance of quality information management, as the integrity of research results directly affects the approval process for medical products. For example, approximately 10-15% of successful 510(k) submissions for Class II products depend on trial information, whereas Class III products necessitate comprehensive trials to prove safety and effectiveness.
Additionally, the World Health Organization (WHO) acknowledges the importance of effective management of medical information, considering that trials frequently involve human participants whose safety is vital. As the landscape of medical device regulation evolves, maintaining high standards in clinical information management becomes increasingly vital for meeting regulatory demands and ensuring market access.
When crafting a Clinical Evaluation Report (CER), it is essential to follow established best practices that prioritize both clarity and thoroughness. A well-structured report begins with a clear introduction that outlines the purpose and scope of the evaluation, setting the stage for the information that follows. Each section should logically flow into the next, detailing the methodology employed, the analysis performed, and the conclusions drawn from the findings.
Utilizing concise and direct language is crucial for effectively communicating results. 'Given the complexities of clinical information, it is vital to present details in a coherent manner, particularly within tables and figures, which serve as critical tools for summarizing information.'. A common challenge in this area is the frequent lack of detailed analysis and consistent presentation, which can create disconnects between the conclusions and the information presented. Ensuring that all claims are supported by robust evidence is not merely a best practice; it is a regulatory necessity, especially in the context of the EU's CE marking requirements.
Furthermore, incorporating post-market monitoring information into your assessment strengthens the thoroughness of the analysis and highlights the significance of ongoing oversight throughout the product lifecycle. This information, frequently overlooked, should be examined with the same attention as primary health-related information to strengthen the safety and efficacy assertions of the medical device.
The significance of a meticulously prepared CER cannot be overstated, as it forms a crucial component of the technical documentation required by regulatory bodies. As pointed out by industry specialists, harmonizing information gathering and analysis approaches across records—such as the assessment strategy and risk management documents—can reduce discrepancies that weaken the overall integrity of the assessment process. By adhering to these principles, one can not only comply with regulatory standards but also contribute to advancing the field of medical technology.

Leveraging templates and checklists can significantly enhance the efficiency of developing Clinical Evaluation Reports (CERs). It is essential to implement standardized CER templates that adhere to regulatory requirements, ensuring that all critical elements are systematically addressed. These elements generally encompass clinical evaluation goals, methodologies, information sources, and conclusions.
Checklists serve as valuable tools in this process, promoting thoroughness and consistency. They help to ensure that every necessary component is included, thus minimizing the risk of oversight. Regular reviews and updates of these templates and checklists are crucial, particularly in light of the evolving regulatory landscape. Adapting to changes not only aids in compliance but also enhances the overall quality of the CER.
In the context of medical device regulations, attention to detail in information analysis and presentation is paramount. As emphasized by industry specialists, a frequent challenge is the gap between the conclusions reached and the actual information provided. Making certain that information stories are consistent and properly coordinated throughout all records—including the assessment strategy, assessment report, and risk management documents—promotes integrity and confidence in the assessment process.
Furthermore, post-market surveillance information, often underutilized, must be analyzed with the same rigor as primary information. The understandings obtained from this information can greatly guide the medical assessment, strengthening the reliability of the CER. By establishing a systematic approach through templates and checklists, organizations can navigate these complexities more effectively, ultimately improving their compliance and efficiency in the clinical evaluation process.

Preparing a Clinical Evaluation Report (CER) entails navigating a myriad of complexities, including information gaps, conflicting evidence, and regulatory compliance challenges that can arise during the process. To skillfully tackle these challenges, it is essential to establish a strong information gathering strategy that not only identifies potential gaps early on but also implements proactive measures to fill them. This can include collaborating with regulatory experts who specialize in navigating the intricate landscape of compliance requirements, ensuring that every aspect of the report adheres to the latest regulations. Open lines of communication with stakeholders are also crucial; engaging with diverse groups throughout the CER development process fosters collaboration and enriches the quality of insights gathered.
A well-structured approach is key, particularly in light of the increasing volume, variety, and velocity of data required for reviews. According to recent studies, ensuring compliance with regulatory bodies has emerged as a top priority for medical device leaders, with 47% highlighting it as a significant challenge. Furthermore, the need for expertise in Regulatory Affairs is paramount, as many organizations face an absence of skills in this area.
Statistical analysis can also play a crucial role, as shown by the Institute for Clinical and Economic Review (ICER), which highlights the significance of evidence-based assessments that include stakeholder input. Engaging interested parties from the outset helps to define the objectives of the data evaluation, allowing for a comprehensive understanding of the context surrounding the clinical evaluation. By employing these strategies, organizations can significantly enhance the reliability and effectiveness of their Clinical Evaluation Reports.

Creating a comprehensive Clinical Evaluation Report (CER) is a multifaceted process that requires a deep understanding of the European Union Medical Device Regulation (EU MDR) requirements. The necessity for manufacturers to demonstrate device safety and performance through rigorous clinical evaluation is paramount. This involves familiarizing oneself with specific regulatory articles, establishing a clear Clinical Evaluation Plan, and ensuring that the evaluation aligns with intended device use and safety expectations.
The clinical evaluation process is structured into distinct stages, including strategic planning, data collection, data analysis, and reporting. Each stage plays a critical role in compiling robust clinical evidence, ensuring that the data is meticulously analyzed, and presented coherently within the CER. By adhering to best practices in writing, utilizing templates and checklists, and addressing common challenges, manufacturers can enhance the quality and integrity of their reports.
In addition, the importance of thorough data management cannot be overstated. The identification and appraisal of clinical data, alongside the integration of post-market surveillance insights, are essential for substantiating claims of safety and effectiveness. By maintaining high standards in data quality and adhering to regulatory requirements, stakeholders can navigate the complexities of medical device regulations more effectively.
Ultimately, a well-constructed CER not only fulfills regulatory obligations but also contributes to the advancement of medical technology and patient safety. By embracing structured methodologies and strategic insights, manufacturers can facilitate successful market entry and ensure ongoing compliance in an ever-evolving regulatory landscape.
What is a Clinical Evaluation Report (CER)?
A Clinical Evaluation Report (CER) is a comprehensive document that assesses the safety and efficacy of medical devices in compliance with the European Union Medical Device Regulation (EU MDR). It is a critical part of the technical documentation required for CE marking and market access in the EU.
Why is a CER important?
A CER is essential not only for regulatory compliance but also for demonstrating the safety and performance of a medical device. It helps manufacturers navigate the complexities of medical regulations and supports successful product certification.
What are the main phases involved in creating a CER?
The creation of a CER generally involves several key phases: Strategic Planning, Data Gathering, Data Analysis, and Reporting.
What role does post-market monitoring play in the CER process?
Post-market monitoring provides critical insights into the ongoing safety and efficacy of medical devices in real-world settings. It should be analyzed rigorously alongside primary health-related information to enhance the overall assessment.
How should clinical information be managed when preparing a CER?
Clinical information management should focus on identifying and appraising relevant studies meticulously. Utilizing appraisal tools like the GRADE system can help assess the quality of evidence and ensure research is conducted ethically and reliably.
What are some best practices for writing a CER?
Best practices include starting with a clear introduction that defines the report's purpose, using concise language and organized sections that flow logically, ensuring that all claims are well-supported by robust evidence, and incorporating post-market surveillance information to strengthen assertions.
How can templates and checklists benefit the CER development process?
Templates and checklists promote efficiency by ensuring that all necessary components are included and systematically addressed. They help maintain thoroughness and consistency, reducing the risk of oversight.
What challenges might arise during the CER preparation?
Common challenges include information gaps, conflicting evidence, and compliance with regulatory standards. Establishing a strong information-gathering strategy and collaborating with regulatory experts can help address these issues effectively.
Why is stakeholder engagement important in the CER process?
Engaging stakeholders fosters collaboration and enriches the quality of insights gathered. It helps define the objectives of the data evaluation and ensures that a comprehensive understanding of the clinical context is achieved.
How does the EU MDR influence the CER process?
The EU MDR establishes rigorous requirements for demonstrating the safety and efficacy of medical devices. Manufacturers must familiarize themselves with specific articles and annexes of the regulation to ensure compliance throughout the evaluation process.