


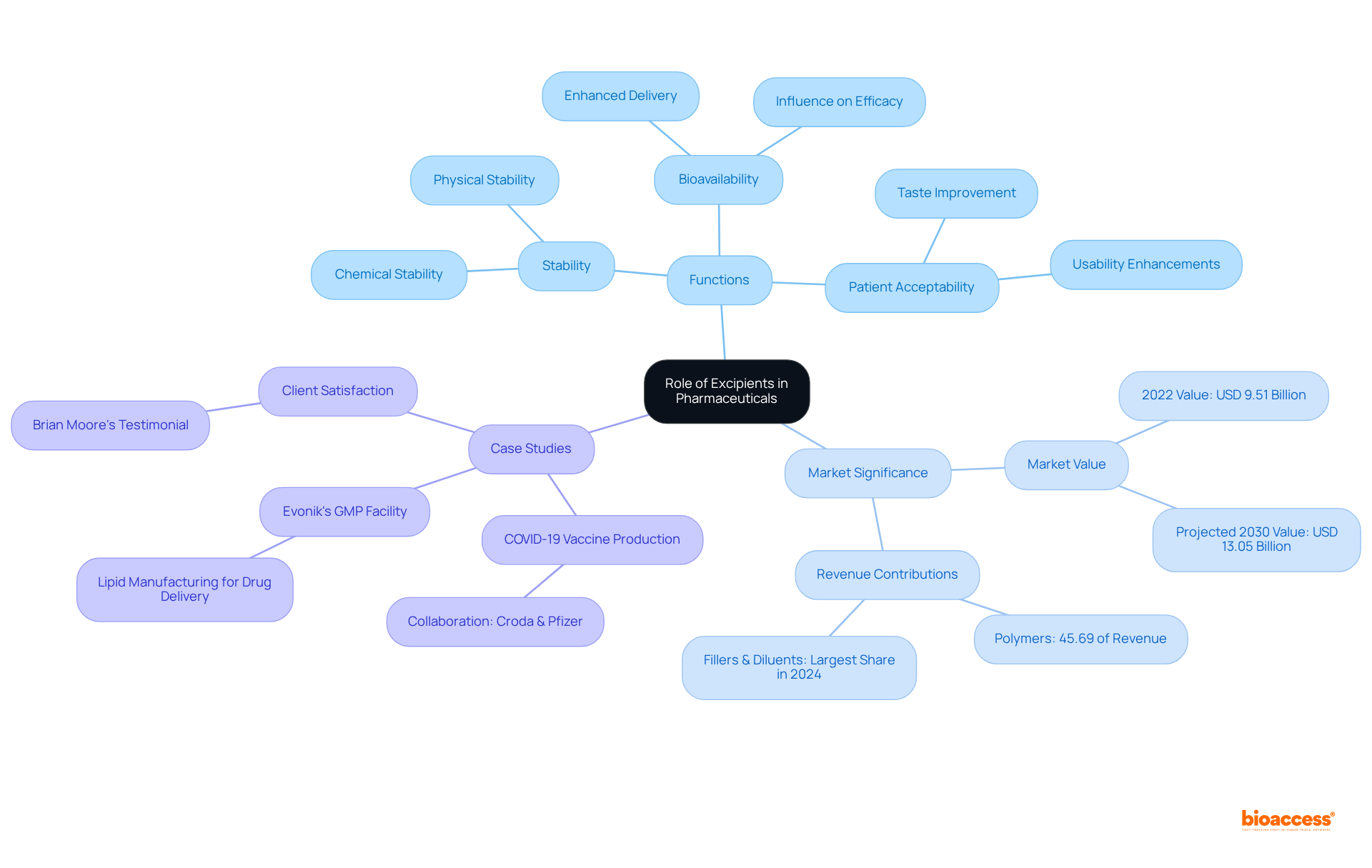
Excipients are vital components in pharmaceutical formulations, serving crucial functions that enhance stability, improve bioavailability, and increase patient acceptability of medications. This article emphasizes their significance by detailing how thoughtfully selected excipients can profoundly influence the efficacy and safety of drugs. Supported by industry growth statistics and case studies, it demonstrates their essential role in successful drug development. Understanding the impact of excipients is not merely academic; it is a call to action for industry stakeholders to prioritize their selection in the formulation process.
Understanding the intricate world of pharmaceuticals reveals that excipients play a pivotal role in drug formulation, acting as the unsung heroes behind medication stability, efficacy, and patient compliance. These inactive ingredients are not merely fillers; they enhance bioavailability, improve taste, and ensure that drugs remain effective throughout their shelf life.
However, the selection and specification of excipients present a complex challenge. How can formulators ensure they choose the right components that meet stringent regulatory standards while also delivering optimal therapeutic outcomes?
This article delves into the essential steps for defining excipients, highlighting their importance and the critical criteria for selecting the right ones in the quest for pharmaceutical success.
To define excipients, it is important to recognize that they are essential components in drug formulations, distinct from the active pharmaceutical ingredient (API), serving multiple critical functions. Their role in stability is paramount; excipients preserve the chemical and physical stability of medications, effectively preventing degradation over time. This stability is crucial for ensuring that medications retain their efficacy throughout their shelf life.
Moreover, excipients enhance the bioavailability of the API, facilitating its delivery to the intended site of action. This aspect is particularly vital, as the selection of inactive ingredients can significantly influence the medication's overall efficacy. Additionally, excipients improve patient acceptability by enhancing the taste, appearance, and usability of medications, making them more palatable and easier for patients to administer. This is essential for adherence to treatment regimens.
The significance of inactive ingredients helps to define excipients, as a poorly chosen component can lead to diminished therapeutic effectiveness or increased side effects. For instance, the global market for pharmaceutical additives, valued at approximately USD 9.51 billion in 2022, is projected to reach USD 13.05 billion by 2030, underscoring the growing recognition of their importance in drug development. Furthermore, polymers, which accounted for over 45.69% of total revenue in 2022, illustrate how additives can provide various functionalities, such as enhanced drug stability and shelf life.
Case studies further demonstrate these effects. During the COVID-19 pandemic, collaborations like that between Croda International Plc and Pfizer Inc. highlighted the essential role of inactive substances in vaccine production, ensuring stability and efficacy during unprecedented times. Additionally, the fillers and diluents segment held the largest share in 2024 due to their crucial role in enhancing formulation bulk and compressibility. Therefore, it is vital for formulators to understand how to define excipients in order to create effective and safe pharmaceutical products.
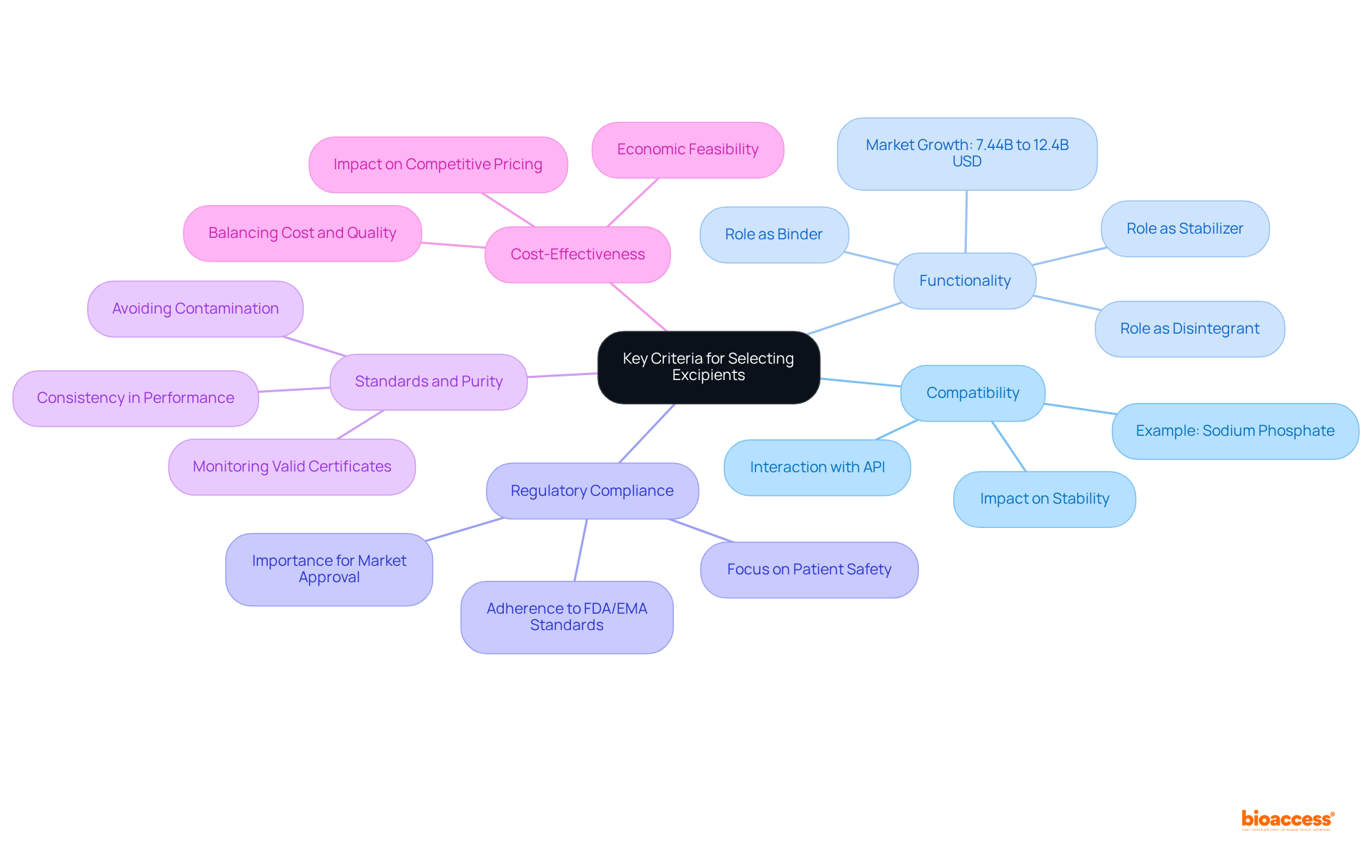
When selecting excipients, it is imperative to consider several key criteria to ensure optimal formulation outcomes:
Compatibility: Confirming that the excipient does not negatively interact with the active pharmaceutical ingredient (API) or other formulation components is essential. Compatibility issues can lead to reduced efficacy or stability of the final product, making this a critical factor in formulation development. For instance, sodium phosphate, the most prevalent additive in biotech-derived medications, underscores the significance of choosing suitable additives.
Functionality: Assessing the substance's capacity to serve its intended purpose—such as functioning as a binder, disintegrant, or stabilizer—is vital. The functionality of additives directly impacts drug performance, influencing factors like release rates and bioavailability. The additives market is projected to expand from 7.44 billion USD in 2024 to 12.4 billion USD by 2035, highlighting the growing significance of proper additive selection.
Regulatory Compliance: It is crucial to ensure that the excipient adheres to necessary regulatory standards for safety and efficacy, as established by organizations such as the FDA and EMA. Compliance with these guidelines is essential for market approval and patient safety. Industry specialists emphasize that the focus on regulatory adherence and assurance of standards is important, as producers seek better substances to satisfy strict safety and effectiveness criteria.
Standards and Purity: Selecting additives that fulfill high benchmarks of standards and purity is necessary to avoid contamination and guarantee consistent performance. High-quality additives contribute to the overall reliability of the pharmaceutical product. Monitoring the count of valid certificates for active pharmaceutical ingredients standards globally highlights the necessity for high-standard additives in formulations.
Cost-Effectiveness: Evaluating the expense of additives in relation to their advantages is crucial, adhering to budgetary limitations without compromising quality. Cost-effective excipients can enhance the economic feasibility of pharmaceutical development while ensuring product integrity. The increasing demand for innovative medication formulations necessitates careful evaluation of excipient expenses to uphold competitive pricing.
By systematically evaluating these criteria, formulators can define excipients that help make informed decisions, significantly enhancing the overall quality and effectiveness of pharmaceutical products. This approach not only aligns with current industry standards but also addresses the growing demand for innovative and effective drug formulations.
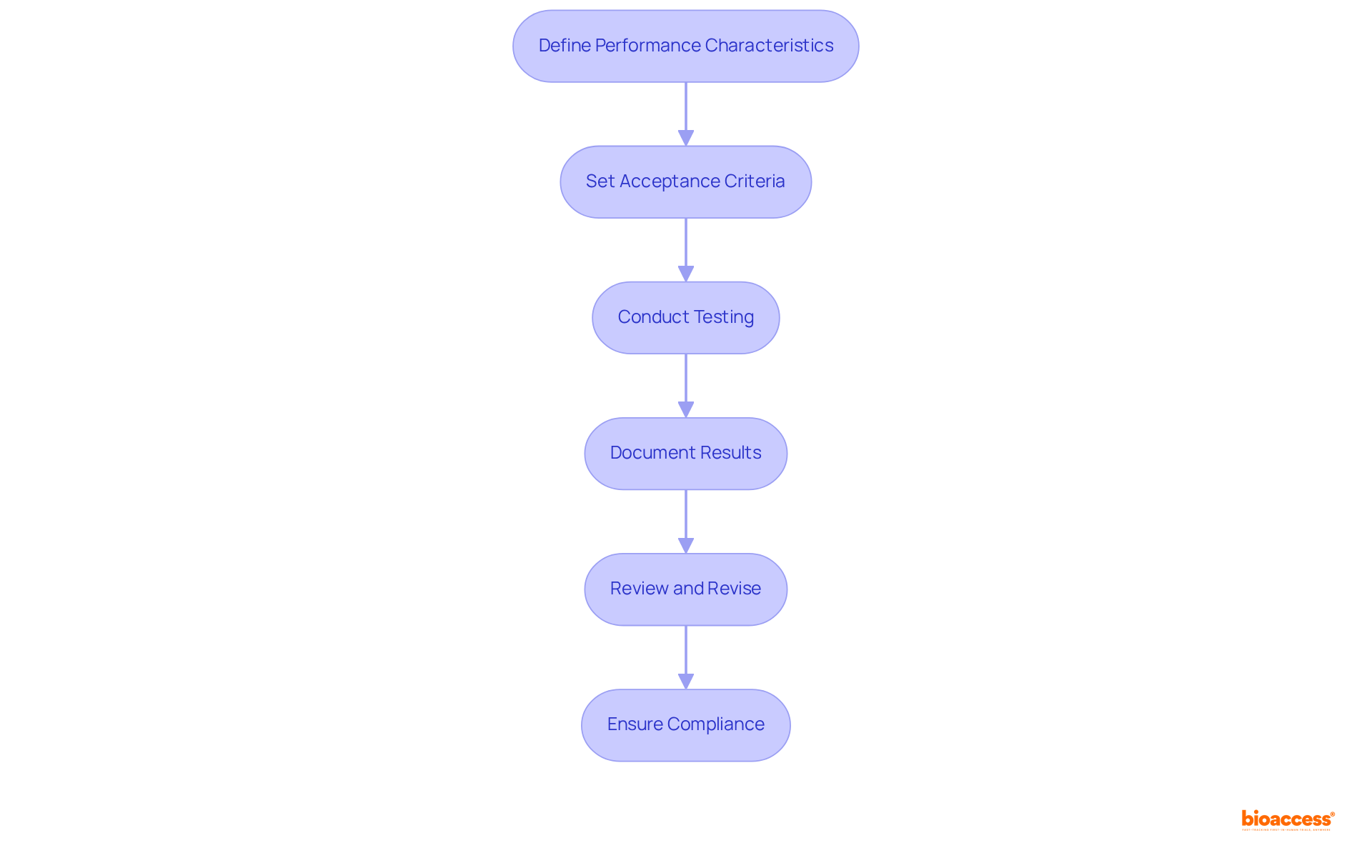
To establish specifications for excipients, it is essential to follow these steps:
Define Performance Characteristics: Begin by identifying the critical performance attributes (CPAs) that are essential for the additive. These include purity, particle size, moisture content, and potential impurities. Understanding these attributes is crucial, as they directly influence the safety and efficacy of the final pharmaceutical product. Notably, there are 42 functional excipient categories listed in USP/NF, which can serve as a valuable guide in the selection process.
Set Acceptance Criteria: Develop specific acceptance criteria for each quality attribute, ensuring that they align with regulatory standards and industry guidelines. For example, the upper specification limit for residual impurities should be calculated based on statistical tolerance intervals, which help ensure that at least 99% of individual values fall within acceptable ranges. The expected failure rate for specification limits stands at 0.0013 (0.13%), underscoring the importance of establishing stringent acceptance criteria for excipients.
Conduct Testing: Implement rigorous testing protocols to evaluate the additive against the established specifications. This includes physical tests (e.g., particle size distribution), chemical analyses (e.g., purity assessments), and microbiological evaluations to ensure the excipient meets all required standards. More than 75% of performance attributes in continued process verification exhibited lag-one sample autocorrelation function estimates between 0.0 and 0.5, highlighting the variability in performance attributes and the necessity for robust testing protocols.
Document Results: Maintain comprehensive documentation of all testing results and specifications to ensure traceability and compliance during regulatory submissions. This documentation should encompass detailed records of testing methodologies, results, and any deviations from expected outcomes.
Review and Revise: Regularly review and update specifications based on emerging research, regulatory changes, or feedback from manufacturing processes. This proactive approach guarantees ongoing compliance and quality assurance, adapting to the evolving landscape of pharmaceutical development.
As Keith M. Bower notes, "Analysts recommended using the sample standard deviation as the estimate of σ when at least 30 results are available for biopharmaceutical CPV activities." By establishing clear specifications, formulators can define excipients to ensure their positive contribution to drug formulations, ultimately enhancing the safety and efficacy of pharmaceutical products.
Excipients are indispensable in the pharmaceutical landscape, acting as vital components that enhance the stability, efficacy, and overall patient experience of medications. Understanding their significance is essential for formulators striving to create safe and effective drug products. By accurately defining excipients, industry professionals ensure that these inactive ingredients fulfill their multiple functions, ultimately contributing to the success of pharmaceutical formulations.
Key points regarding the selection and specification of excipients have been emphasized throughout this article. Critical criteria guiding the selection process include:
Furthermore, establishing robust specifications through performance characteristics and rigorous testing guarantees that excipients meet necessary quality benchmarks, which is vital for patient safety and product integrity.
The significance of excipients cannot be overstated in the ongoing pursuit of innovative and effective drug formulations. As the pharmaceutical industry continues to evolve, so must the strategies for excipient selection and specification. By prioritizing high-quality excipients and adhering to established guidelines, formulators can markedly enhance the therapeutic effectiveness of medications. Embracing these practices not only aligns with industry standards but also addresses the increasing demand for safe and efficacious pharmaceutical products, ultimately improving patient outcomes and adherence to treatment regimens.
What are excipients in pharmaceuticals?
Excipients are essential components in drug formulations that are distinct from the active pharmaceutical ingredient (API). They serve multiple critical functions, including preserving the stability of medications and enhancing the bioavailability of the API.
Why is the stability of excipients important?
The stability of excipients is crucial because they help maintain the chemical and physical stability of medications, preventing degradation over time and ensuring that medications retain their efficacy throughout their shelf life.
How do excipients affect the bioavailability of medications?
Excipients enhance the bioavailability of the API, facilitating its delivery to the intended site of action, which significantly influences the overall efficacy of the medication.
In what ways do excipients improve patient acceptability?
Excipients improve patient acceptability by enhancing the taste, appearance, and usability of medications, making them more palatable and easier for patients to administer, which is essential for adherence to treatment regimens.
What can happen if excipients are poorly chosen?
A poorly chosen excipient can lead to diminished therapeutic effectiveness or increased side effects, negatively impacting the medication's overall performance.
What is the projected growth of the pharmaceutical additives market?
The global market for pharmaceutical additives was valued at approximately USD 9.51 billion in 2022 and is projected to reach USD 13.05 billion by 2030, indicating a growing recognition of the importance of excipients in drug development.
What role do polymers play in excipients?
Polymers accounted for over 45.69% of total revenue in 2022 and provide various functionalities, such as enhanced drug stability and shelf life.
Can you provide an example of the importance of excipients in real-world applications?
During the COVID-19 pandemic, collaborations like that between Croda International Plc and Pfizer Inc. highlighted the essential role of inactive substances in vaccine production, ensuring stability and efficacy during unprecedented times.
Which segment of excipients held the largest share in 2024?
The fillers and diluents segment held the largest share in 2024 due to their crucial role in enhancing formulation bulk and compressibility.
Why is it vital for formulators to understand excipients?
It is vital for formulators to understand how to define excipients in order to create effective and safe pharmaceutical products.