


The article defines a medical device as a healthcare instrument specifically designed for diagnosis, prevention, monitoring, treatment, or alleviation of illness, which does not primarily achieve its intended effects through chemical action. This definition underscores the diverse range of medical devices, from simple tools to complex technologies, highlighting their critical role in healthcare innovation and regulatory compliance. Understanding this definition is essential for grasping the broader implications of medical technology in clinical research and practice.
Understanding the intricate landscape of medical devices is essential in today’s rapidly evolving healthcare environment. These instruments, which range from simple tongue depressors to advanced imaging machines, play a pivotal role in diagnosis, treatment, and patient monitoring. With the global market for medical devices projected to reach an astounding $678.88 billion by 2025, grasping their defining characteristics and regulatory frameworks becomes increasingly vital.
However, given the diverse classifications and stringent compliance requirements, how can manufacturers and healthcare stakeholders effectively navigate the complexities of medical device definitions while ensuring the safety and efficacy of their products?
A healthcare instrument encompasses a broad spectrum of tools, apparatuses, machines, appliances, implants, reagents for in vitro use, software, and materials specifically designed for health-related purposes. This category ranges from fundamental instruments like tongue depressors to sophisticated devices such as pacemakers and diagnostic imaging machines. The definition of a medical device encompasses its defining characteristics, which include:
For instance, the diagnostic imaging sector, which constitutes approximately 20% of the overall healthcare equipment market, exemplifies the critical role these tools play in health services. Moreover, in-vitro diagnostics account for around 10% of the market, underscoring their importance in disease detection and management.
Industry leaders highlight the significance of these characteristics for regulatory compliance and product development. Specialists assert that understanding the intended application and classification of healthcare instruments is vital, especially as the global market is projected to reach $678.88 billion by 2025, growing at a compound annual growth rate (CAGR) of 6%. This expansion is driven by technological advancements and the increasing demand for advanced healthcare solutions.
Practical examples of healthcare tools include wearable health monitors that facilitate real-time tracking of vital signs, significantly enhancing patient care. These instruments not only improve health outcomes but also align with the growing trend towards personalized medicine. As the healthcare technology landscape evolves, remaining informed about these essential characteristics is crucial for stakeholders within the healthcare sector.

The oversight environment for healthcare products is intricate and varies significantly across regions, with major entities such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) playing crucial roles. In the United States, the FDA categorizes medical devices into three classes based on risk:
Each category is subject to specific regulatory requirements that manufacturers must meet to ensure safety and effectiveness. For instance, Class III products, often comprising implantable devices, require rigorous pre-market approval processes, including clinical trials to demonstrate their safety and efficacy.
Conversely, the European Union's Medical Device Regulation (MDR) establishes a more stringent framework that not only mirrors the classification system but also imposes additional responsibilities for clinical evaluation and post-market surveillance. Under the MDR, manufacturers are required to submit comprehensive clinical data to substantiate their claims, thereby ensuring that products adhere to high safety and performance standards. Recent updates to the MDR, effective from May 2021, have further underscored the significance of post-market monitoring and risk management, aligning with global trends toward enhanced patient safety.
As the medical equipment industry continues to evolve, regulatory changes are anticipated in 2025, particularly concerning orphan medical products and high-risk classifications. The EMA has initiated a pilot program aimed at assisting manufacturers in navigating these complexities, offering free guidance on orphan product status and clinical evaluation strategies. This initiative is essential for devices intended for life-threatening conditions or those aimed at pediatric populations, ensuring that innovative solutions can reach the market more efficiently.
Understanding these compliance structures is vital for companies seeking to introduce healthcare devices that align with the definition of a medical device into the marketplace. Compliance not only facilitates legal operations but also protects patient safety, highlighting the necessity for manufacturers to remain informed about evolving regulations and best practices. bioaccess® offers professional services that streamline the clinical trial process, including feasibility studies, investigator selection, and regulatory compliance, which are crucial for addressing the challenges faced by medical technology startups. Engaging proactively with these frameworks, supported by bioaccess's comprehensive project management and reporting services, is essential for successful market entry and sustained operational integrity.
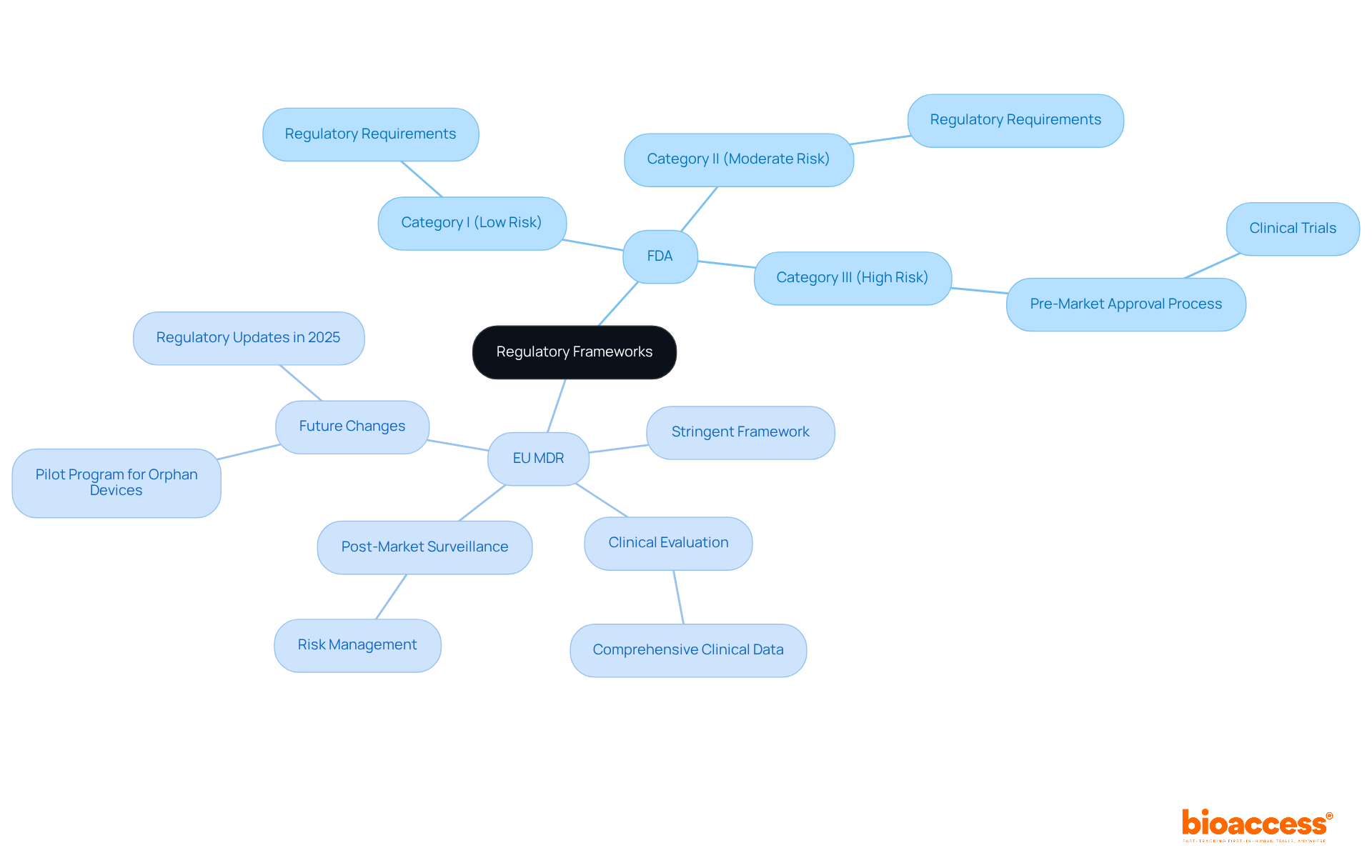
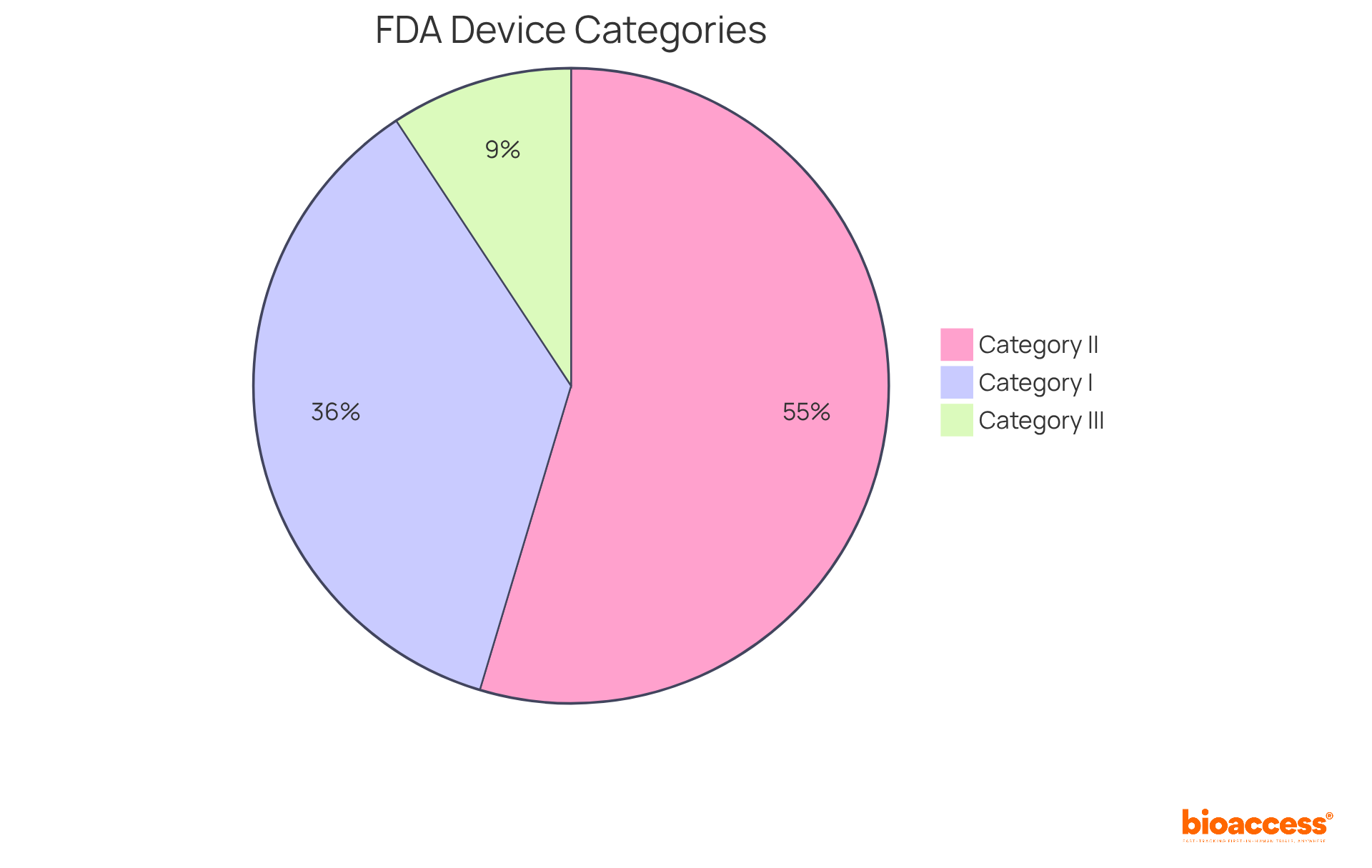
Medical instruments are classified according to the definition of a medical device, which considers their intended application and the associated risk levels they pose to patients. The FDA categorizes these instruments into three primary groups:
Category I products, which constitute approximately 35% of all controlled medical instruments, are typically low-risk and subject to limited oversight. Examples include adhesive bandages and tongue depressors. In contrast, Group II instruments, accounting for around 53% of authorized items, require more rigorous supervision and must meet specific performance criteria. Common examples include infusion pumps and powered wheelchairs. Finally, Category III items, which make up roughly 9% of authorized products, are high-risk and include implantable devices such as cardiac pacemakers and breast implants. These products face the most stringent compliance standards due to their potential for significant harm, necessitating thorough premarket authorization procedures to demonstrate safety and efficacy.
In the European Union, the classification system has evolved to encompass four categories:
Each is defined by similar risk-based criteria. Understanding these classifications is crucial for manufacturers, as they dictate the essential testing, documentation, and approval processes required for market entry, which are all influenced by the definition of a medical device. The FDA emphasizes that the classification system informs the appropriate oversight measures for ensuring safe and effective market availability, thereby guaranteeing that products are thoroughly evaluated in accordance with their risk levels.
In Latin America, particularly in Colombia, the oversight framework is managed by INVIMA (Colombia National Food and Drug Surveillance Institute), which plays a vital role in the examination and monitoring of health-related products. INVIMA's Directorate for Medical Devices and other Technologies ensures compliance with health standards and oversees both pre- and post-market activities. This regulatory body is recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, underscoring its expertise in ensuring the safety, efficacy, and quality of healthcare products in the region. For companies navigating clinical trials, collaborating with experts like bioaccess can provide comprehensive clinical trial management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, ensuring a streamlined process from early feasibility studies to post-market follow-up.
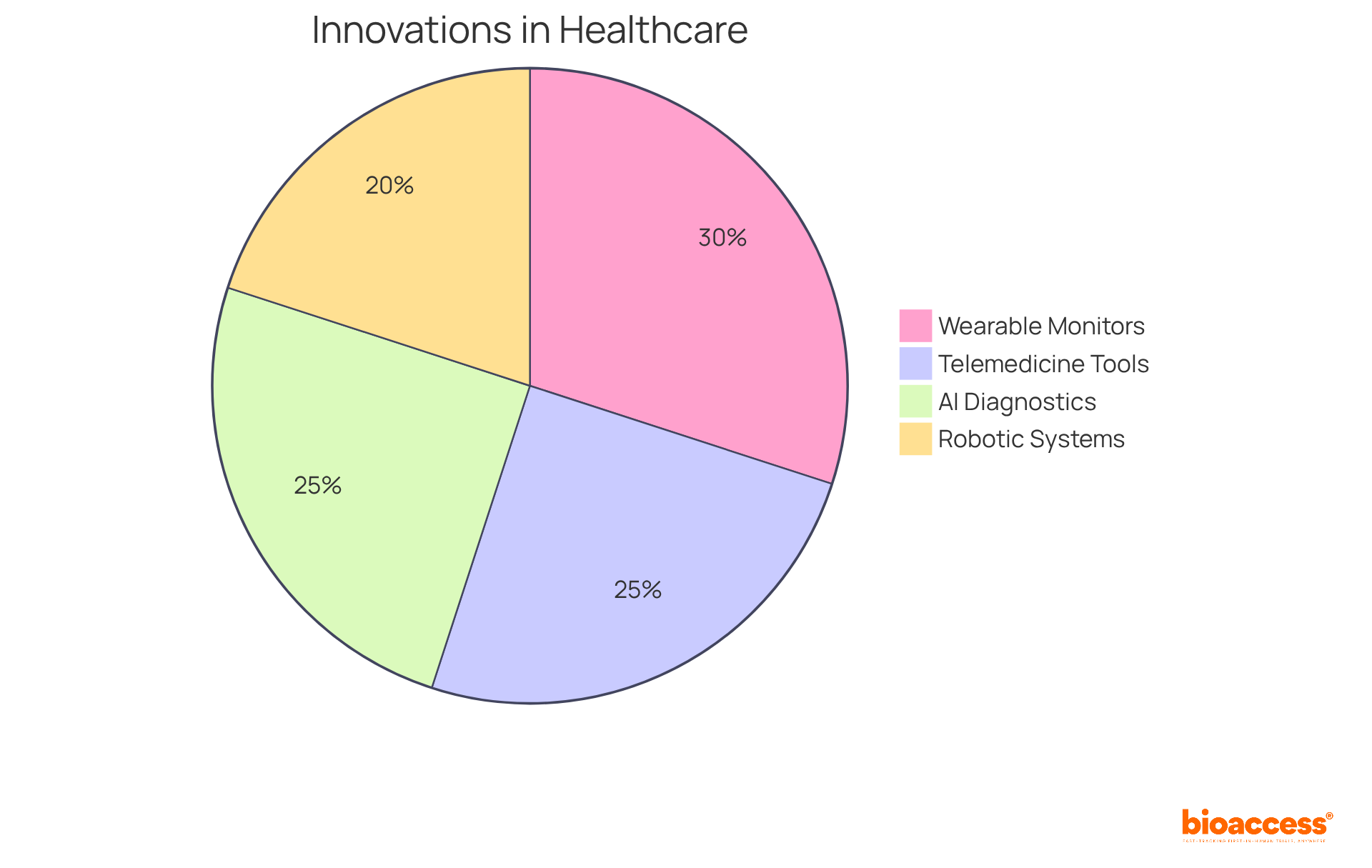
Healthcare instruments are pivotal in driving innovation within the healthcare sector, significantly enhancing the ability to diagnose, treat, and manage a range of health conditions. At bioaccess™, we are committed to advancing medical technologies through innovation and quality, contributing to the evolution of healthcare. Innovations such as wearable health monitors, telemedicine tools, and robotic surgical systems have transformed patient care by facilitating real-time monitoring and minimally invasive procedures. These advancements not only enhance patient outcomes but also improve efficiency within healthcare systems.
For instance, the integration of artificial intelligence in diagnostic tools has led to faster and more accurate diagnoses, with 69% of healthcare organizations either evaluating or utilizing AI technologies, ultimately saving lives and reducing healthcare costs. The global wearable healthcare market is projected to grow from USD 34.9 billion in 2022 to approximately USD 156 billion by 2032, reflecting a significant trend towards the adoption of these technologies. Moreover, 86% of patients report that wearable healthcare technology tools improve health outcomes, underscoring their positive impact on patient care.
As the healthcare equipment sector continues to evolve, expected to reach a market size of $678.88 billion by 2025, the ongoing development of innovative technologies will be crucial in addressing the challenges faced by healthcare professionals and enhancing the quality of care provided to patients. Leaders in healthcare assert that these technological innovations not only improve patient outcomes but also boost operational efficiency within healthcare systems, emphasizing the definition of a medical device in contemporary medicine. Notably, advancements in vascular medicine, such as those presented by Dr. Jorge Hernando Ulloa at the Charing Cross International Symposium, illustrate the influence of clinical research in fostering innovation and regulatory excellence in Latin America.
The exploration of what constitutes a medical device reveals a complex yet essential framework that underscores its significance in healthcare. By defining medical devices through their intended use and non-pharmaceutical nature, it becomes clear that these instruments play a critical role in diagnosis, treatment, and patient care. Their classification based on risk levels, as established by regulatory bodies like the FDA and EMA, further emphasizes the importance of safety and efficacy in the development of healthcare technologies.
Key insights discussed throughout the article highlight the diversity of medical devices, from simple tools to advanced technologies, and the regulatory challenges that accompany their introduction to the market. The growth of the medical device market, projected to reach nearly $679 billion by 2025, is driven by technological advancements and a rising demand for innovative healthcare solutions. Additionally, the role of regulatory frameworks in ensuring compliance and patient safety cannot be overstated, as they guide manufacturers in navigating the complexities of medical device development.
As the healthcare landscape continues to evolve, understanding and adhering to these definitions and classifications becomes paramount for stakeholders in the industry. Engaging with innovative technologies and regulatory guidelines not only enhances patient outcomes but also fosters a culture of safety and quality in healthcare. The future of medical devices lies in the hands of those who prioritize these principles, ensuring that advancements in health technology lead to improved care and innovation in the medical field.
What is a medical device?
A medical device is a healthcare instrument that includes a wide range of tools, apparatuses, machines, appliances, implants, reagents for in vitro use, software, and materials specifically designed for health-related purposes.
What are the core characteristics of a medical device?
The core characteristics of a medical device include its intended use for diagnosis, prevention, monitoring, treatment, or alleviation of illness, and its non-pharmaceutical nature, meaning it does not achieve its primary intended effects through chemical action or metabolic processes.
Can you provide examples of medical devices?
Examples of medical devices range from basic instruments like tongue depressors to advanced technologies such as pacemakers and diagnostic imaging machines.
What percentage of the healthcare equipment market is made up of diagnostic imaging and in-vitro diagnostics?
The diagnostic imaging sector constitutes approximately 20% of the overall healthcare equipment market, while in-vitro diagnostics account for around 10%.
Why is understanding the characteristics of medical devices important?
Understanding the characteristics of medical devices is vital for regulatory compliance and product development, particularly as the global market is projected to grow significantly, reaching $678.88 billion by 2025.
What is driving the growth of the medical device market?
The growth of the medical device market is driven by technological advancements and the increasing demand for advanced healthcare solutions.
How do wearable health monitors fit into the category of medical devices?
Wearable health monitors are practical examples of medical devices that facilitate real-time tracking of vital signs, enhancing patient care and aligning with the trend towards personalized medicine.