


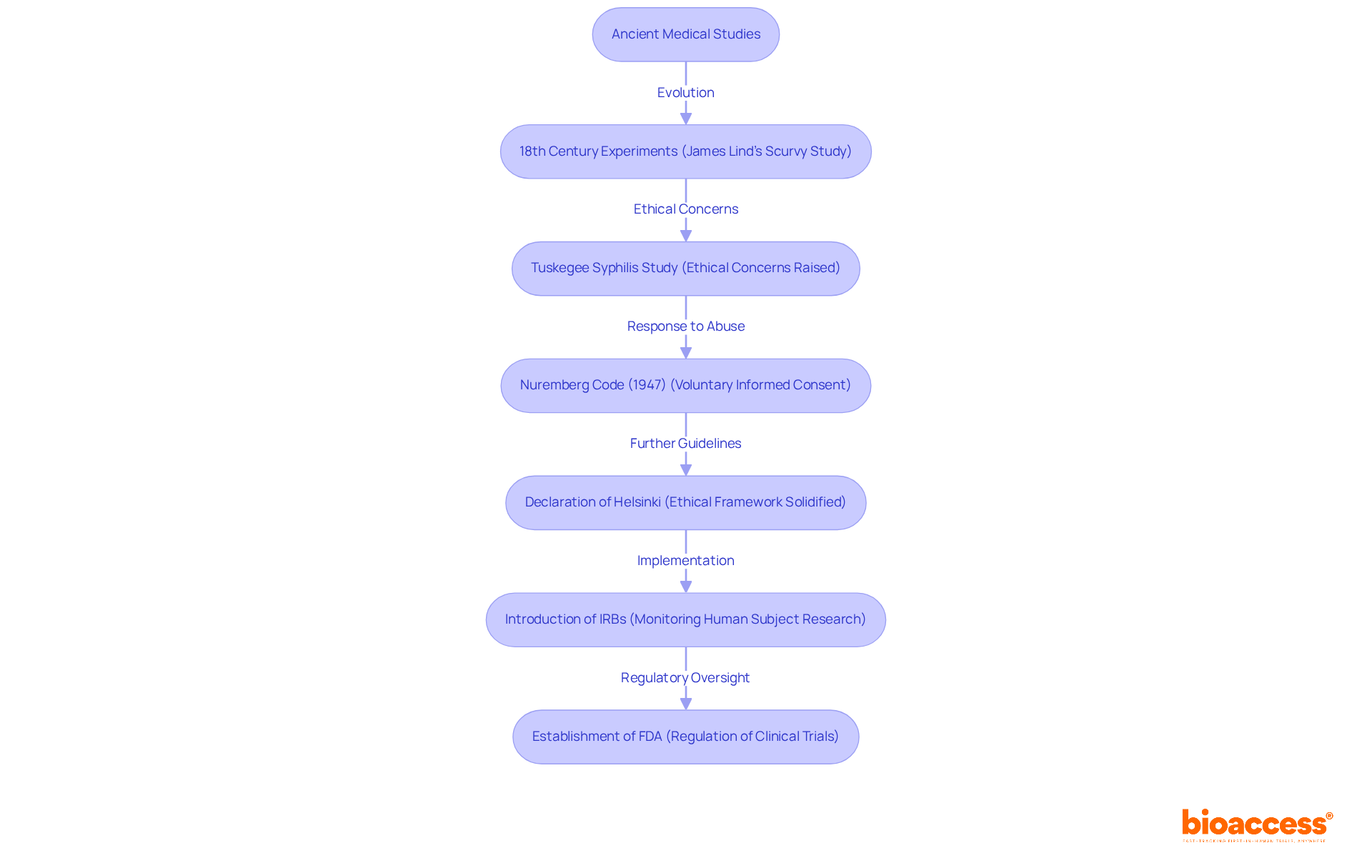
The article defines clinical research as a systematic exploration of health and disease through human participants, underscoring its pivotal role in evaluating the safety and efficacy of new treatments, drugs, and medical devices. This definition is bolstered by an exploration of the historical evolution, ethical guidelines, and the various phases of clinical research. Each aspect highlights the critical importance of clinical research in advancing medical science and enhancing patient care. By understanding these elements, readers can appreciate the collaborative efforts necessary to navigate the complexities of the Medtech landscape and address key challenges effectively.
The realm of clinical research serves as the backbone of modern medicine, intertwining human health and scientific inquiry to unlock new treatments and therapies. By systematically investigating the safety and efficacy of medical innovations, clinical research not only enhances patient care but also drives significant advancements in healthcare practices.
However, as the field evolves, questions arise:
This article delves into the definition, importance, and key characteristics of clinical research, exploring its pivotal role in shaping the future of medicine.
The definition of clinical research represents a vital segment of medical inquiry, focusing on the systematic exploration of health and disease through human participants. The definition of clinical research includes the primary objective of assessing the safety and efficacy of new treatments, drugs, and medical devices. This study is essential for improving medical understanding and care for individuals, as it provides the evidence necessary to inform the definition of clinical research and medical practices. The definition of clinical research involves involving human participants in medical investigations, effectively linking laboratory findings with real-world applications to ensure that new treatments are both safe and effective for public use.
Effective medical studies have shown substantial enhancements in patient care. For instance, advancements in methodologies such as decentralized studies have accelerated participant recruitment and increased diversity among study populations, addressing the common issue of under-enrollment, which affects approximately 37% of research sites. Moreover, the adoption of electronic data capture systems has streamlined data management, enhancing regulatory compliance and efficiency.
The advantages of medical studies go beyond specific experiments; they aid in wider healthcare progress. Clinical trials represent almost 40% of the U.S. pharmaceutical funding budget, amounting to approximately $7 billion each year, with a significant portion designated for participant recruitment. This investment highlights the essential function of medical studies in creating groundbreaking treatments that can change health results.
Expert views emphasize the fundamental importance of the definition of clinical research in the field of healthcare. It not only aids in assessing new therapies but also guides medical guidelines and practices, ultimately resulting in enhanced care statistics. For instance, involvement in medical trials has been demonstrated to improve treatment choices and results for individuals, as indicated by the increased participation in medical studies noted in England, where over 952,000 people took part in the 2022/23 timeframe, showcasing a rebound and greater interest in medical investigations after the pandemic.
In summary, medical studies are a cornerstone of contemporary medicine, driving advancements that significantly influence patient care and healthcare practices. At this point, bioaccess® rises as a prominent contract study organization (CRO) in Latin America, offering affordable and high-quality services customized for Medtech startups. With a dedication to speeding up study outcomes, bioaccess® guarantees swift regulatory approval, efficient site activation, and effective participant recruitment, thereby improving the overall investigative landscape in the region.
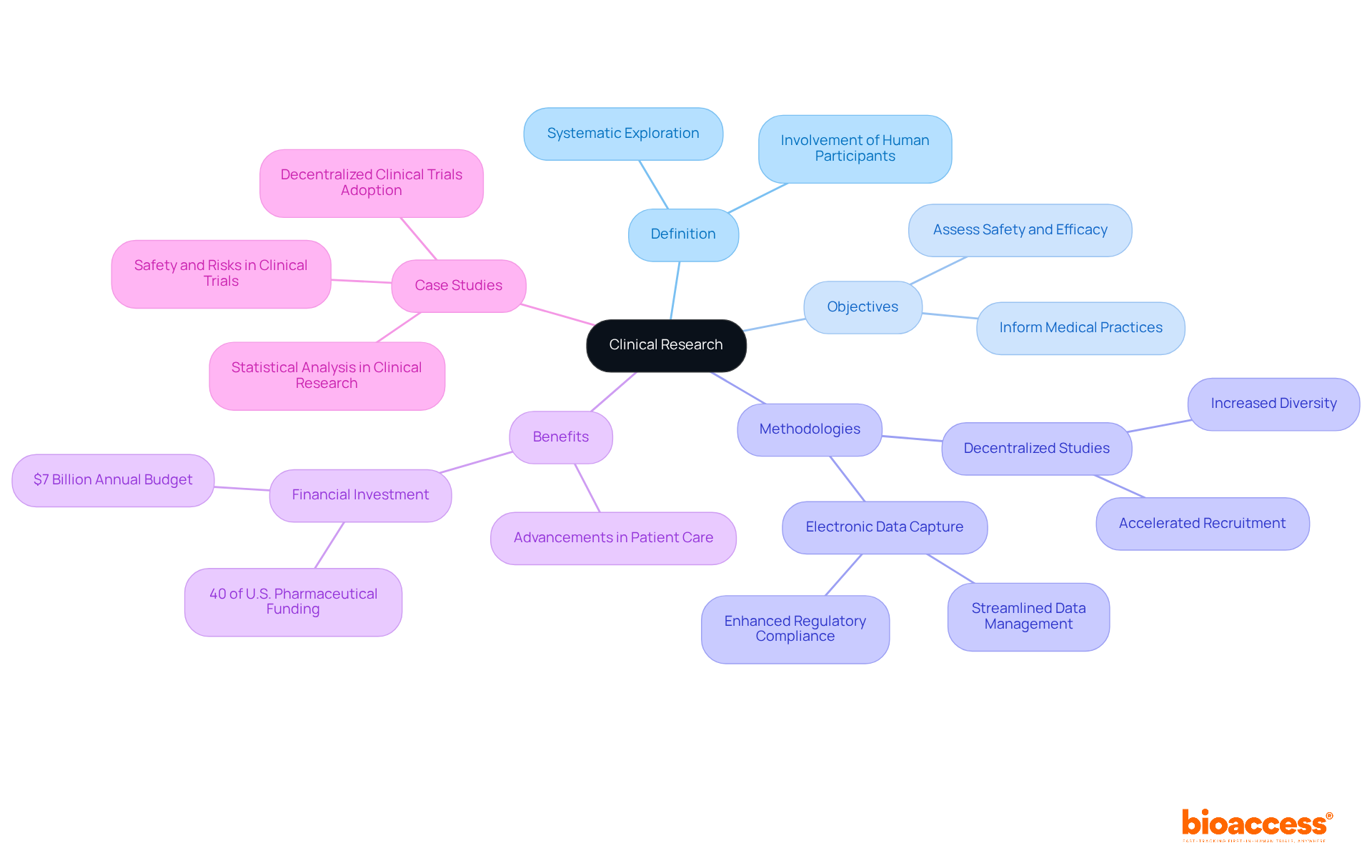
The history of medical studies stretches back to ancient times, with early references found in biblical texts and the writings of Hippocrates. However, the contemporary era of medical investigation truly began in the 18th century, marked by regulated experiments such as James Lind's examination of scurvy in sailors. Over the years, the field has evolved significantly, particularly in response to ethical concerns stemming from historical abuses, such as the Tuskegee Syphilis Study.
This evolution prompted the establishment of ethical guidelines, including the Nuremberg Code in 1947, which underscored the necessity of voluntary informed consent and the protection of human subjects' welfare. The Declaration of Helsinki further solidified these principles, shaping the ethical framework of medical studies.
The definition of clinical research indicates that medical studies are governed by stringent guidelines designed to protect the safety and rights of participants; notably, 75% of studies include both males and females, while 12% involve children. Key milestones in this evolution include:
As the field continues to advance, the integration of ethical guidelines remains paramount, fostering a culture of respect, transparency, and accountability in medical studies.
The definition of clinical research includes a systematic approach characterized by several distinct phases.
Adherence to Good Clinical Practice (GCP) guidelines is essential throughout these phases. The definition of clinical research includes the ethical and scientific quality standards outlined by GCP for designing, conducting, and reporting research studies. This framework not only safeguards participant welfare but also enhances the credibility of findings, ensuring they can be reliably generalized to larger populations.
Current compliance rates with GCP guidelines serve as crucial indicators of study integrity, reflecting the commitment of investigators to uphold high standards in medical research. For instance, completion rates for clinical trials are approximately:
This underscores the demanding nature of clinical studies. Moreover, as digital compliance becomes increasingly significant, understanding user preferences, such as cookie management, is vital for maintaining ethical standards in all investigative practices.

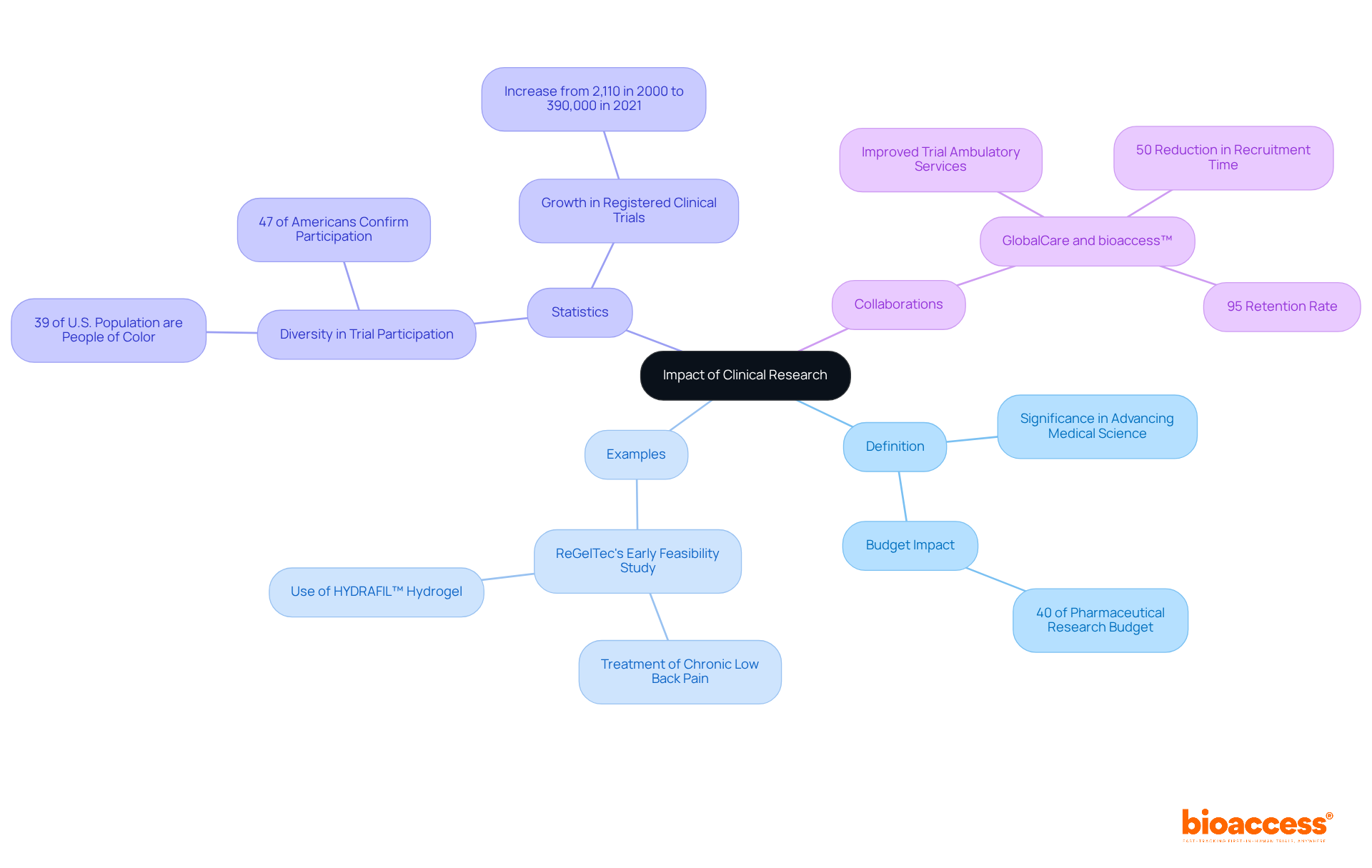
The definition of clinical research indicates that clinical studies are essential in advancing medical science and enhancing individual care. Rigorous testing of new therapies has resulted in significant breakthroughs, including the development of life-saving medications and innovative treatment protocols. A notable example is ReGelTec's Early Feasibility Study in Colombia, where eleven patients suffering from chronic low back pain were successfully treated with HYDRAFIL™, a patented hydrogel that is melted before injection into the nucleus of a degenerated disc via a 17-gauge needle. This research not only demonstrates the promise of new therapies but also highlights the efficiency of remote supervision in medical studies.
Moreover, the collaboration between GlobalCare Clinical Trials and bioaccess™ has significantly improved trial ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and an impressive 95% retention rate. These advancements underscore the definition of clinical research, illustrating how medical studies can effectively address critical health needs and enhance access to innovative treatments. By consistently evaluating and refining medical techniques, studies ensure that healthcare professionals can deliver the most efficient and secure interventions to those they serve.
Importantly, the definition of clinical research indicates that medical studies account for nearly 40% of the pharmaceutical research budget in the United States, underscoring their financial significance. Furthermore, the increasing diversity in clinical trial participation is crucial for ensuring that new treatments are effective across various populations, ultimately leading to improved patient outcomes.
The exploration of clinical research underscores its critical role in advancing medical science and enhancing patient care. By systematically investigating health and disease through human participation, clinical research assesses the safety and efficacy of new treatments while bridging the gap between laboratory findings and real-world applications. This essential process guarantees that innovative therapies are both effective and safe, ultimately improving healthcare outcomes for individuals.
Key insights from the discussion illuminate the historical evolution of clinical research, the ethical frameworks established to protect participants, and the structured phases that guide the research process. From the early experiments of the 18th century to today's stringent guidelines, clinical research has undergone significant transformation, reflecting a commitment to ethical standards and participant welfare. The substantial financial investment in clinical trials highlights their importance, with nearly 40% of the pharmaceutical budget allocated to these studies, facilitating groundbreaking advancements in treatment options.
In light of these findings, it is evident that clinical research serves as a cornerstone of modern medicine. Stakeholders in healthcare, including researchers, practitioners, and policymakers, must continue to support and engage in clinical studies to foster innovation and ensure that diverse populations benefit from new treatments. By prioritizing participation in clinical trials, individuals can contribute to the ongoing quest for improved health outcomes, reinforcing the significance of clinical research in shaping the future of healthcare.
What is the definition of clinical research?
Clinical research is the systematic exploration of health and disease through human participants, primarily aimed at assessing the safety and efficacy of new treatments, drugs, and medical devices.
Why is clinical research important?
Clinical research is crucial for improving medical understanding and care, as it provides the evidence necessary to inform medical practices and ensures that new treatments are safe and effective for public use.
How do clinical trials impact patient care?
Effective medical studies, including clinical trials, have demonstrated significant enhancements in patient care by improving treatment choices and outcomes, as well as accelerating the development of new therapies.
What methodologies have improved participant recruitment in clinical research?
Advancements such as decentralized studies have accelerated participant recruitment and increased diversity among study populations, addressing issues of under-enrollment at research sites.
How much funding is allocated to clinical trials in the U.S.?
Clinical trials represent almost 40% of the U.S. pharmaceutical funding budget, amounting to approximately $7 billion each year, with a significant portion designated for participant recruitment.
What role do clinical studies play in healthcare progress?
Clinical studies not only assess new therapies but also guide medical guidelines and practices, contributing to wider healthcare advancements and improved care statistics.
How has participation in medical studies changed recently?
There has been increased participation in medical studies, particularly noted in England, where over 952,000 people participated in the 2022/23 timeframe, indicating a rebound and greater interest in medical investigations after the pandemic.
What is bioaccess® and what services do they provide?
Bioaccess® is a prominent contract study organization (CRO) in Latin America that offers affordable and high-quality services tailored for Medtech startups, focusing on speeding up study outcomes and improving the investigative landscape in the region.