


Successfully designing trials for wearables in Bolivia requires:
It is crucial to have thorough knowledge of local regulations, as well as to engage with stakeholders, to ensure compliance and promote participant diversity. Furthermore, strong data management practices are essential for enhancing the credibility of trial results.
Navigating the landscape of wearable trials in Bolivia presents a unique set of challenges and opportunities for researchers and companies alike. As the demand for innovative health solutions grows, understanding the regulatory framework becomes crucial for success.
Identifying key regulatory bodies and ensuring compliance with international standards are vital steps in the trial process. Furthermore, defining clear objectives, designing robust protocols, and implementing effective recruitment strategies significantly enhance the quality and diversity of participant pools.
By establishing comprehensive data management and analysis protocols, researchers can ensure the credibility of their findings. This article delves into these critical aspects, providing a roadmap for conducting successful wearable trials in Bolivia.
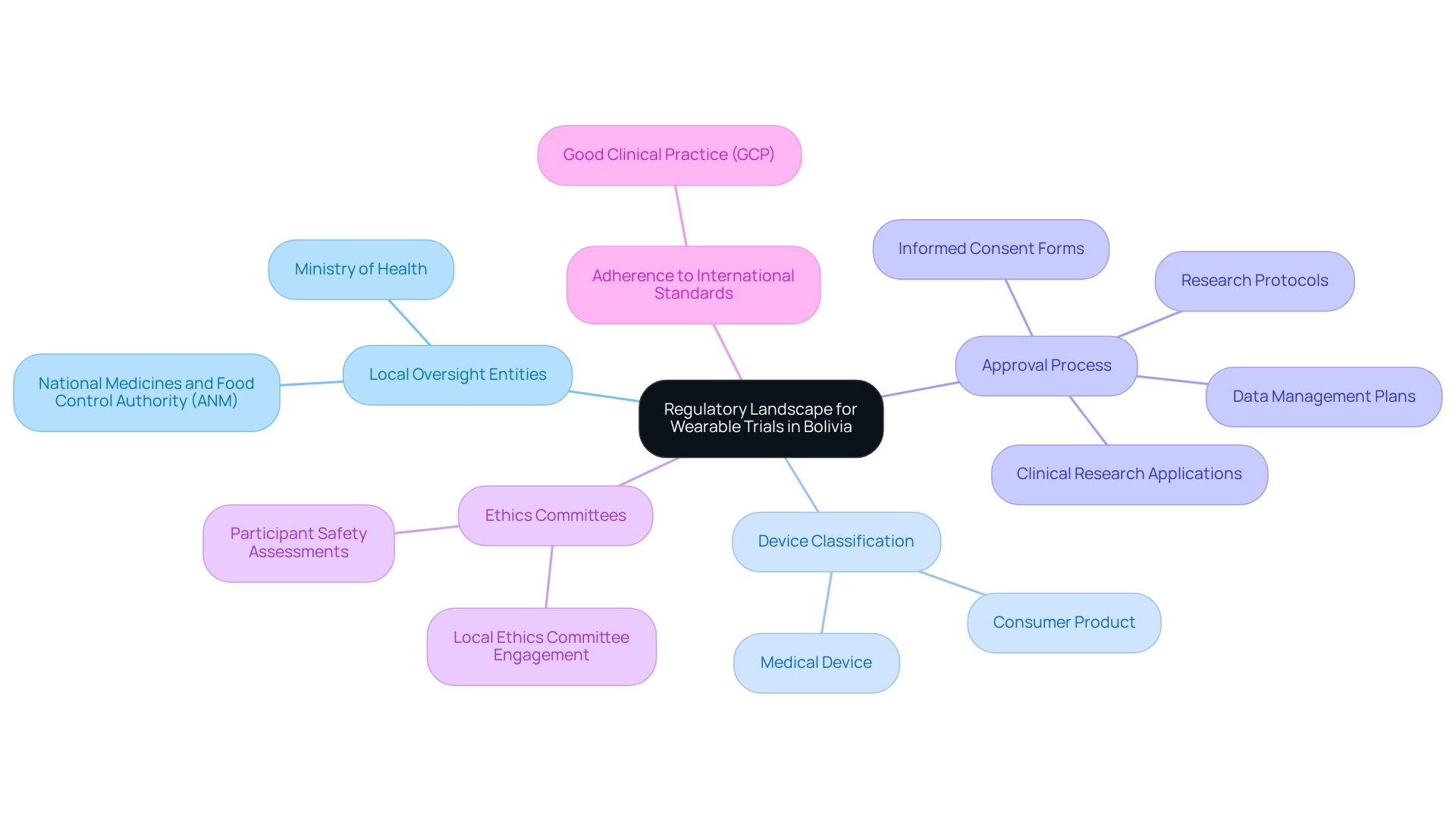
To effectively carry out designing trials for wearables in Bolivia, it is crucial to familiarize yourself with the following essential compliance aspects:
Local Oversight Entities: Identify the primary governing authorities in Bolivia, such as the National Medicines and Food Control Authority (ANM) and the Ministry of Health. Understanding their roles will facilitate navigation through the approval process.
Device Classification: Ascertain whether your wearable device is classified as a medical device or a consumer product. This classification significantly influences the regulatory pathway and the requirements for clinical trials.
Approval Process: Prepare for the submission of clinical research applications, which typically include comprehensive research protocols, informed consent forms, and data management plans. Ensure that your documentation aligns with the specific requirements established by Bolivian authorities.
Ethics Committees: Engage with local ethics committees early in the process to secure necessary approvals. These committees assess the ethical implications of your study and ensure participant safety.
Adherence to International Standards: Align your study with international guidelines such as Good Clinical Practice (GCP) to enhance credibility and facilitate smoother compliance interactions.
In addition to these oversight components, leveraging comprehensive clinical study management services can significantly streamline your process. bioaccess® offers expertise in study setup, project management, compliance evaluations, and several crucial studies, including Early-Feasibility Studies and First-In-Human Studies. By thoroughly understanding these regulatory components and utilizing specialized services, you can establish a solid foundation for your device assessment in Bolivia.
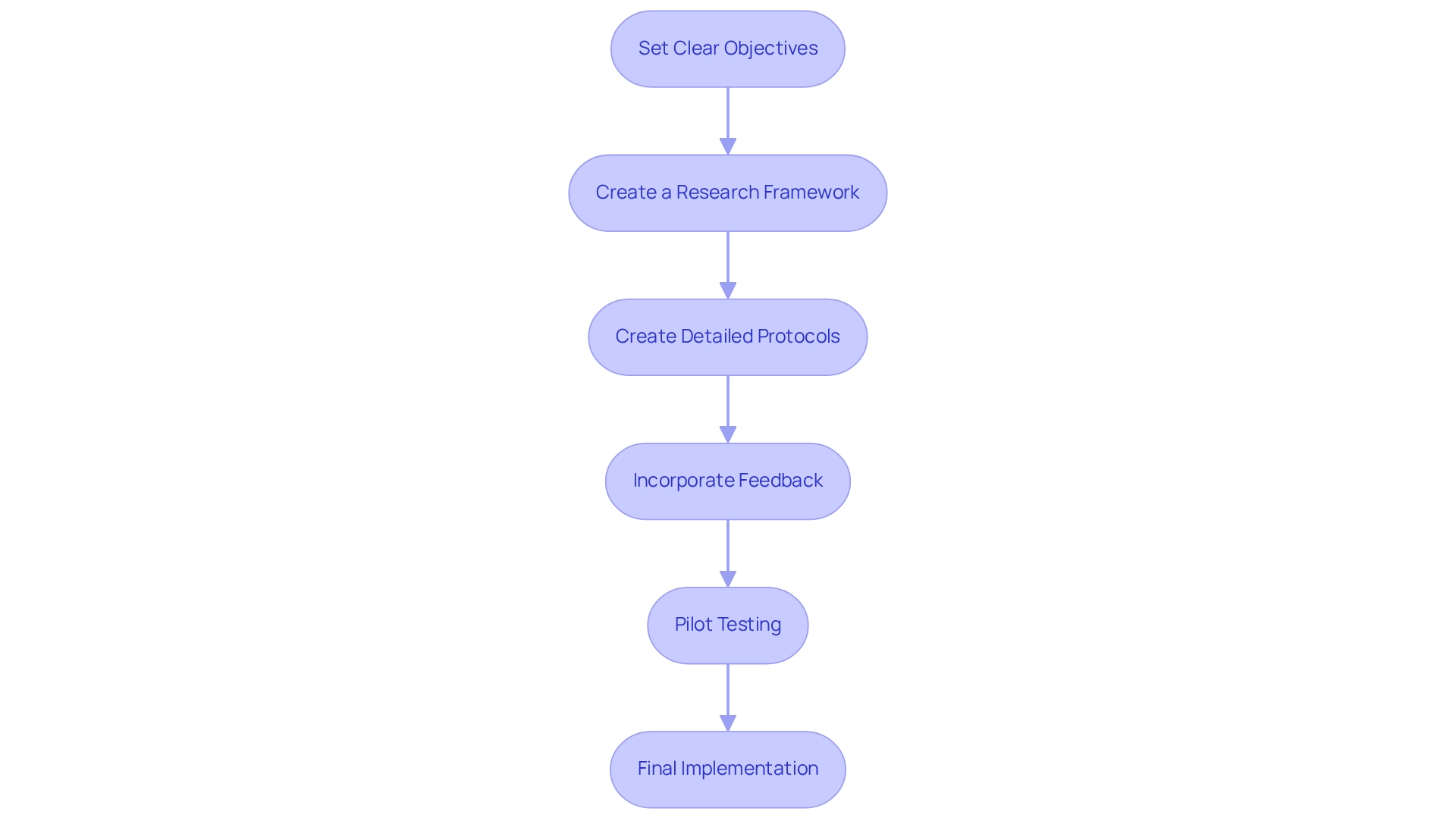
To effectively define objectives and design protocols for your device experiment, follow these steps:
Set Clear Objectives: Establish specific, measurable, achievable, relevant, and time-bound (SMART) objectives. For instance, if your wearable device aims to monitor heart rate, specify the target population and the expected outcomes.
Create a Research Framework: Select a suitable research design (e.g., randomized controlled trial, observational analysis) according to your objectives. Consider factors such as sample size, duration, and control groups. Utilizing bioaccess®'s expertise in Early-Feasibility, First-In-Human, Pilot, and Post-Market Clinical Follow-Up research can provide valuable insights into designing trials for wearables in Bolivia tailored for the Latin American market.
Create Detailed Protocols: Draft comprehensive protocols that outline the study's methodology, including participant selection criteria, intervention details, and data collection methods. Ensure that these protocols align with legal requirements, utilizing bioaccess®'s knowledge of compliance reviews and trial setup to streamline this process.
Incorporate Feedback: Engage with stakeholders, including oversight organizations and ethics committees, to review and refine your protocols. Their insights can help identify potential issues early in the process. Bioaccess® can assist in navigating these discussions, ensuring that your protocols meet local regulatory expectations.
Pilot Testing: Conduct a pilot trial to assess the feasibility of your protocols. This step enables you to recognize any logistical challenges and implement necessary modifications before the comprehensive test. With bioaccess®'s extensive project management services, you can efficiently oversee pilot testing and guarantee a seamless shift to larger experiments. By carefully outlining your goals and creating solid protocols, while utilizing the thorough clinical research management services provided by bioaccess®, including Pilot and Post-Market Clinical Follow-Up assessments, you improve the chances of significant outcomes when designing trials for wearables in Bolivia.
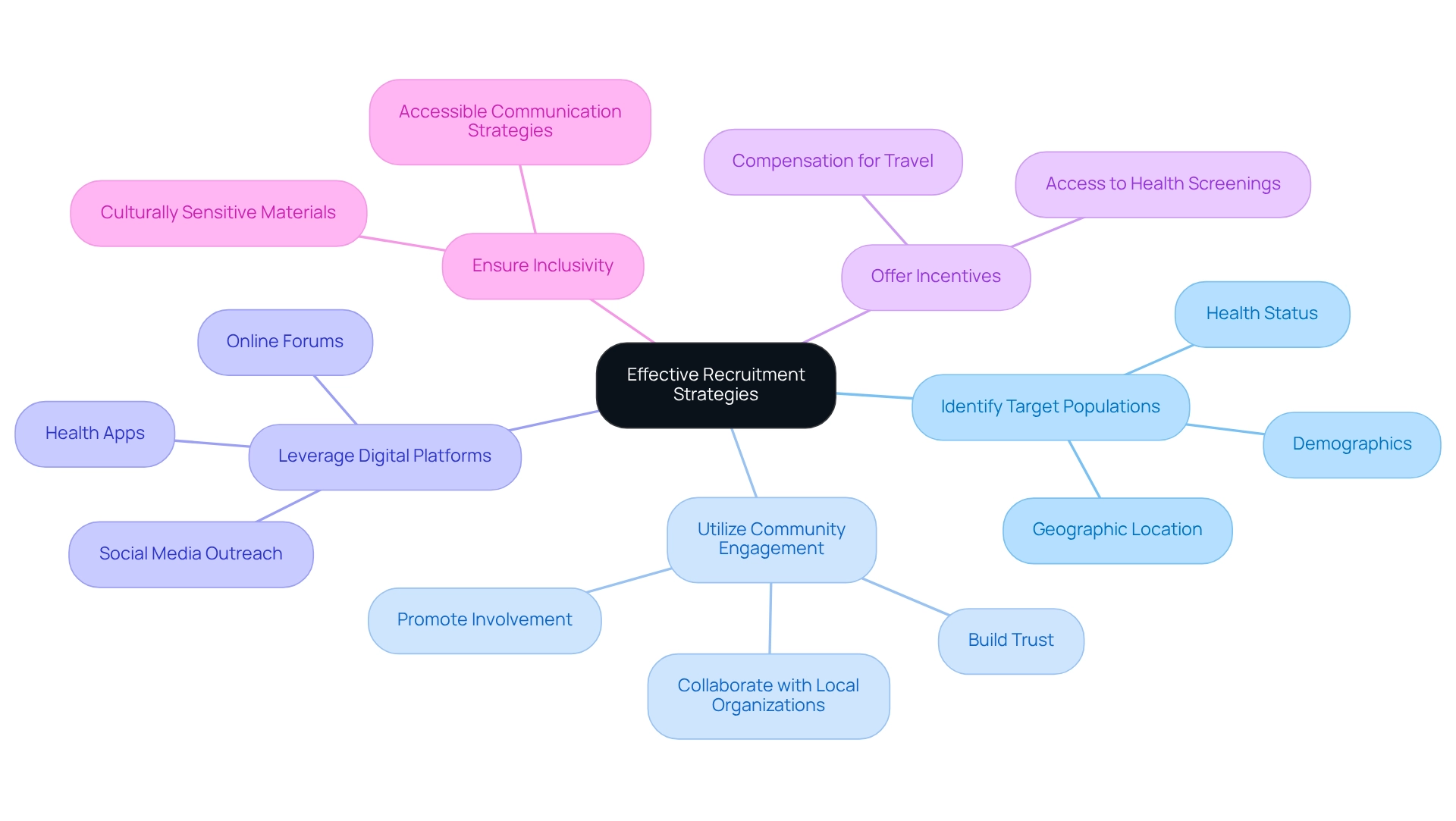
To implement effective recruitment strategies for your wearable study, consider the following approaches:
Identify Target Populations: Define the demographics of your target participants based on the objectives of your study. Consider factors such as age, gender, health status, and geographic location.
Utilize Community Engagement: Collaborate with local organizations, healthcare providers, and community leaders, such as those involved in the partnership between bioaccess™ and Caribbean Health Group, to increase awareness about your study. Interacting with the community builds trust and promotes involvement, which is crucial for establishing Barranquilla as a prominent location for clinical research in Latin America.
Leverage Digital Platforms: Use social media, online forums, and health apps to reach potential participants. Digital outreach can help you connect with a broader audience and facilitate enrollment.
Offer Incentives: Consider providing incentives for participation, such as compensation for travel expenses or access to health screenings. Incentives can motivate individuals to join the study and complete follow-up assessments.
Ensure Inclusivity: Develop materials and communication strategies that are culturally sensitive and accessible to diverse populations. This method aids in removing obstacles to involvement and promotes a more inclusive research atmosphere.
By utilizing these recruitment tactics, you can increase participant diversity and enhance the overall quality of your study, ultimately supporting the success of clinical research efforts in Colombia.
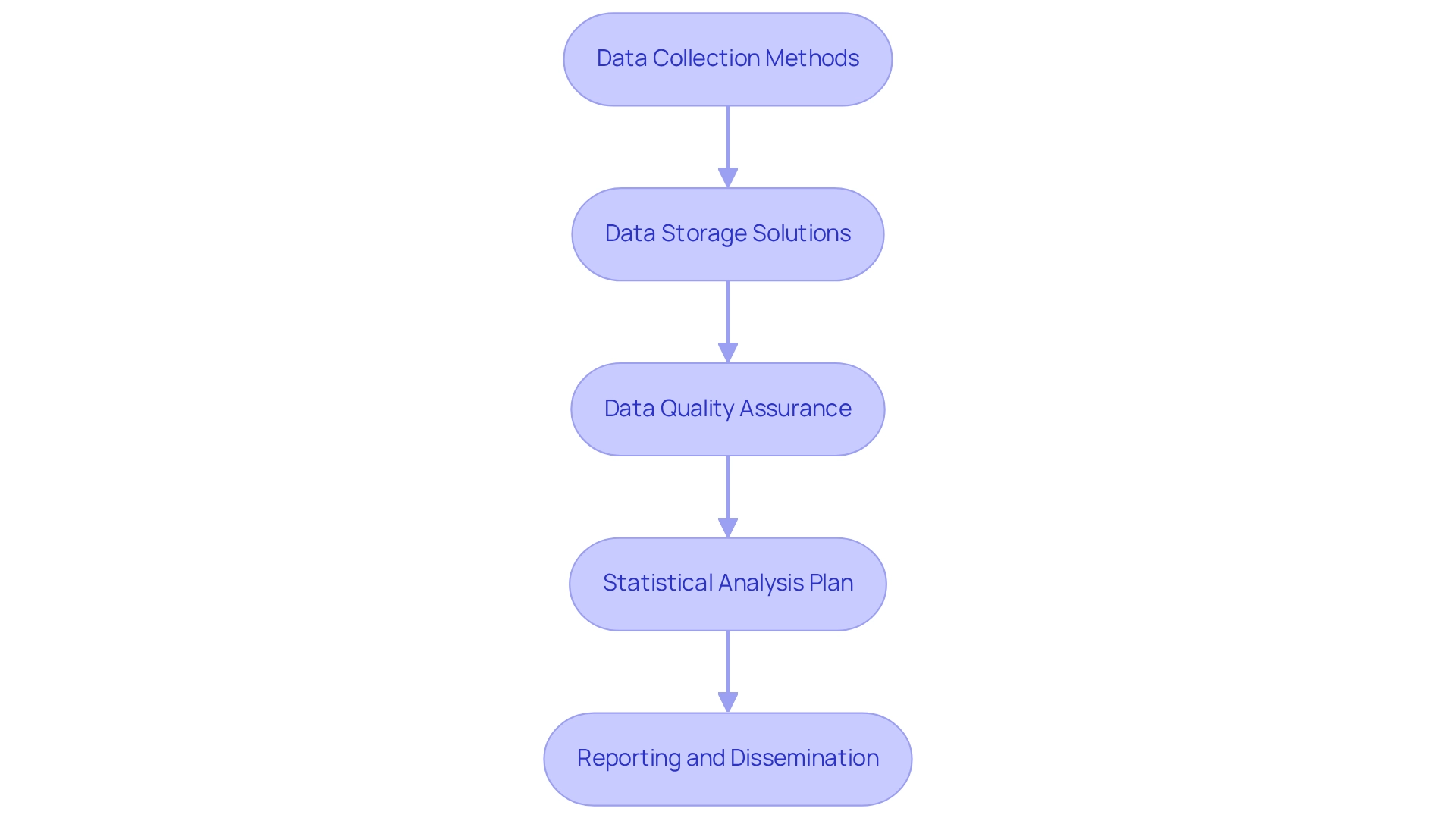
To establish effective data management and analysis protocols for your wearable trial, adhere to the following guidelines:
Data Collection Methods: Clearly define the methods for collecting data from wearable devices. It is essential that these methods are reliable and capable of capturing the necessary metrics, such as heart rate and activity levels.
Data Storage Solutions: Select secure data storage solutions that comply with local regulations and protect participant privacy. Utilizing encrypted databases and secure cloud storage options is advisable. At bioaccess®, we prioritize data protection and have established grievance procedures to address any concerns regarding the processing of participant information. Should you have any queries or concerns, please contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), located at 1200 Brickell Avenue, Suite 1950 #1034, or via email at info@bioaccessla.com.
Data Quality Assurance: Implement robust quality control measures to ensure data accuracy and completeness. Regular audits of data collection processes should be conducted, and any discrepancies must be addressed promptly.
Statistical Analysis Plan: Develop a comprehensive statistical analysis plan that outlines the methods for analyzing data to meet study objectives. Clearly specify the statistical methods and software that will be utilized.
Reporting and Dissemination: Formulate a plan for how results will be reported and disseminated to stakeholders, including regulatory bodies and the scientific community. It is crucial that findings are communicated transparently and responsibly. bioaccess® is dedicated to addressing client concerns in accordance with relevant laws, ensuring compliance and transparency throughout the research process.
Enhancing the credibility and reliability of your wearable trial results is crucial when designing trials for wearables in Bolivia, which can be achieved by establishing comprehensive data management and analysis protocols.
Successfully navigating wearable trials in Bolivia necessitates a profound understanding of the regulatory landscape, clear objectives, effective recruitment strategies, and robust data management protocols. Familiarity with local regulatory bodies, device classifications, and approval processes is crucial for compliance and seamless trial execution. Engaging with ethics committees and aligning with international standards, such as Good Clinical Practice, further enhances the credibility of the research.
Defining specific objectives and designing detailed protocols are essential steps that guide the trial's direction and methodology. Incorporating stakeholder feedback and conducting pilot studies refines these protocols, thereby increasing the likelihood of achieving meaningful results. Effective recruitment strategies targeting diverse populations and fostering community engagement enhance participant diversity, which is vital for the success of clinical research initiatives.
Lastly, establishing comprehensive data management and analysis protocols ensures the reliability and validity of collected data. By prioritizing data quality, security, and transparency, researchers uphold ethical standards and disseminate findings responsibly. Collectively, these strategies provide a roadmap for conducting successful wearable trials in Bolivia, ultimately contributing to the advancement of innovative health solutions in the region.
What are the primary governing authorities for wearables in Bolivia?
The primary governing authorities in Bolivia include the National Medicines and Food Control Authority (ANM) and the Ministry of Health.
How does the classification of a wearable device affect regulatory compliance?
The classification of a wearable device as either a medical device or a consumer product significantly influences the regulatory pathway and the requirements for clinical trials.
What documentation is required for the approval process of clinical trials in Bolivia?
The approval process typically requires the submission of clinical research applications, which include comprehensive research protocols, informed consent forms, and data management plans that align with Bolivian authorities' specific requirements.
Why is it important to engage with local ethics committees?
Engaging with local ethics committees early in the process is crucial to secure necessary approvals, as these committees assess the ethical implications of the study and ensure participant safety.
What international standards should be adhered to when conducting studies in Bolivia?
Studies should align with international guidelines such as Good Clinical Practice (GCP) to enhance credibility and facilitate smoother compliance interactions.
How can clinical study management services assist in the process?
Leveraging comprehensive clinical study management services can significantly streamline the process, offering expertise in study setup, project management, compliance evaluations, and conducting crucial studies like Early-Feasibility Studies and First-In-Human Studies.