


This article outlines the crucial steps for effectively designing trials for wearable devices in Peru, highlighting the significance of:
It elaborates on the regulatory landscape governed by the Peruvian National Institute of Health, the necessity of informed consent, and the imperative for structured data collection and analysis processes. These elements are essential to ensure ethical compliance and enhance the credibility of trial outcomes.
Navigating the world of wearable technology trials presents a complex endeavor, particularly within the intricate regulatory landscape of Peru. As health tech advances rapidly, comprehending the legal framework surrounding clinical research becomes crucial for success. The Peruvian National Institute of Health plays a vital role in trial approvals, emphasizing the importance of informed consent and data protection. For researchers aiming to innovate responsibly, the stakes are high. Organizations must strive to design effective and compliant trials through a structured approach that incorporates:
This article explores the critical components that ensure successful wearable device trials, offering insights to streamline the process and enhance outcomes in the ever-evolving healthcare landscape.
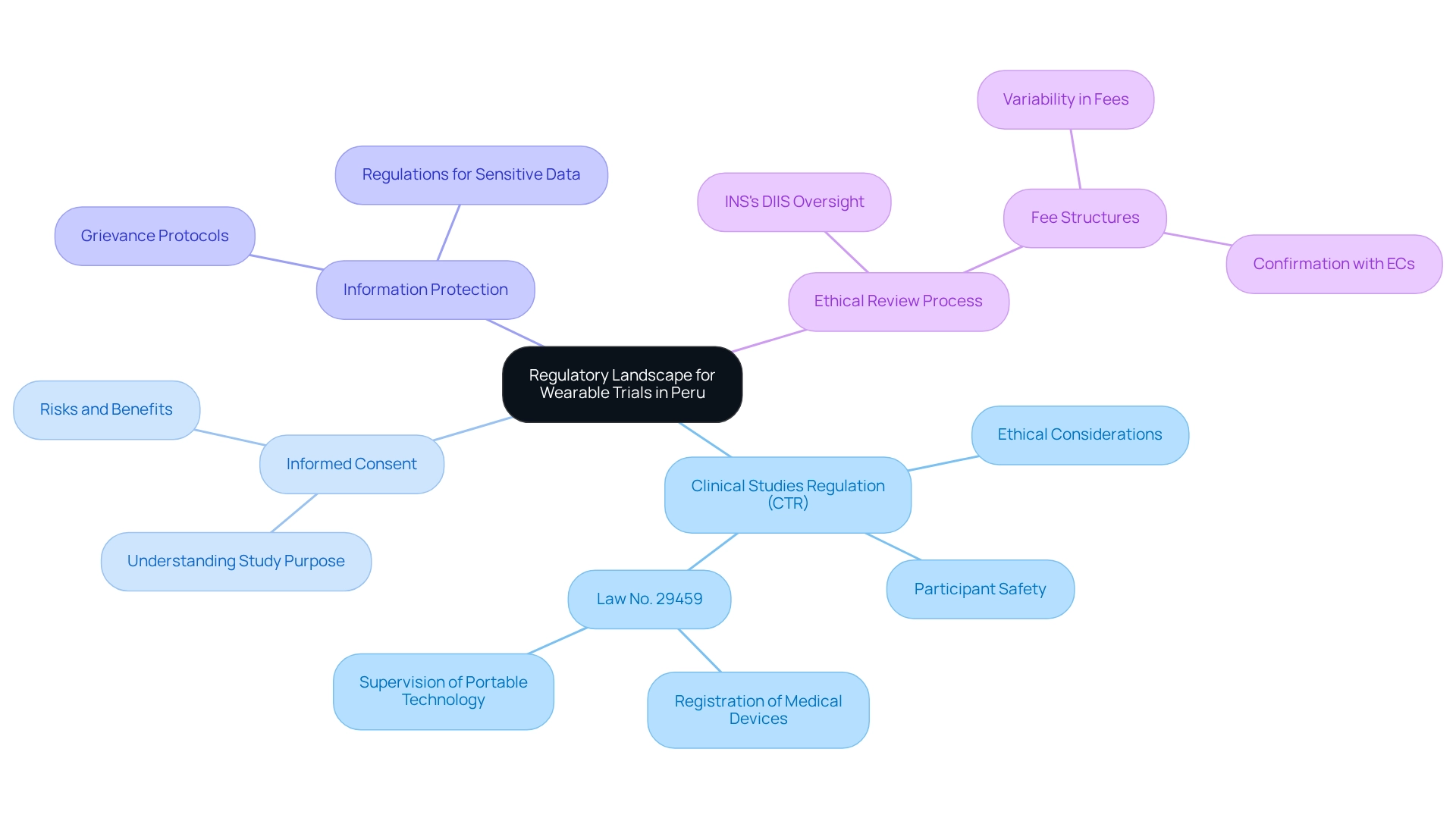
Designing trials for wearables in Peru requires effectively navigating the regulatory framework governing clinical research. Begin by reviewing the guidelines established by the Peruvian National Institute of Health (INS), which oversees clinical study approvals and compliance. Key regulations include:
Furthermore, the INS's DIIS supervises the ethical review process for clinical studies, highlighting the importance of ethical adherence in your study design. Understanding the fee structures related to Institutional Ethics Committee evaluations can provide valuable insights into potential expenses and fluctuations in the ethics review process, which is crucial for designing trials for wearables in Peru, enhancing your study design and mitigating potential compliance challenges. For further insights, refer to resources such as the ClinRegs database and the official INS website, which offer comprehensive information on the regulatory environment in Peru. Leveraging the expertise of bioaccess®, specializing in Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and Post-Market Clinical Follow-Up Studies (PMCF), can further streamline your clinical study processes, ensuring a successful transition from pilot study to commercialization.
Establishing clear goals is essential when designing trials for wearables in Peru. Here are essential steps to guide the process:
By following these steps, you can create a strong structure for your wearable test, which is essential when considering designing trials for wearables in Peru, significantly enhancing the likelihood of achieving meaningful and impactful results. Successful experiments often combine quantitative and qualitative methods, enhancing patient identification and engagement. Furthermore, compliance with ethical and regulatory standards, including informed consent and participant safety, not only protects participant rights but also bolsters the credibility of your trial outcomes, fostering trust in the research process. For example, adhering to ethical guidelines is essential, as shown in the case analysis titled 'Following Ethical and Regulatory Standards,' which demonstrates how compliance improves credibility and trust in research results. Additionally, remember that formal approval of the SAP from the study sponsor or steering committee is required before distribution to stakeholders, ensuring that all procedural steps are followed.
To effectively manage and analyze data, designing trials for wearables in Peru requires adherence to the following protocols:
Successfully navigating the regulatory landscape for wearable technology trials in Peru necessitates a comprehensive understanding of the guidelines from the Peruvian National Institute of Health. Key regulations, such as the Clinical Studies Regulation and Law No. 29459, are essential for ensuring compliance, participant safety, and ethical considerations, including informed consent and data protection.
Establishing clear objectives and designing appropriate parameters are critical for conducting effective trials. Engaging stakeholders and conducting pilot studies not only help refine approaches but also enhance the relevance of results. A combination of quantitative and qualitative methods improves patient engagement and ensures adherence to ethical standards, thereby increasing the credibility of outcomes.
Moreover, implementing robust data management and analysis protocols is vital for maintaining the integrity of findings. This involves a solid data collection strategy, secure storage, and comprehensive statistical analysis. Continuous monitoring and transparent reporting of results, whether positive or negative, are also crucial for advancing wearable technology in healthcare.
In conclusion, a structured approach emphasizing regulatory compliance, clear objectives, and effective data management is imperative for successful wearable technology trials in Peru. By focusing on these elements, researchers can adeptly navigate the complexities of clinical trials, drive innovation, and build trust within the healthcare landscape, ultimately enhancing trial outcomes and fostering responsible advancements in health tech.
What is the primary organization overseeing clinical research regulations in Peru?
The primary organization is the Peruvian National Institute of Health (INS), which oversees clinical study approvals and compliance.
What is the Clinical Studies Regulation (CTR)?
The Clinical Studies Regulation (CTR) outlines the requirements for conducting clinical studies in Peru, emphasizing ethical considerations and participant safety.
What does Law No. 29459 regulate?
Law No. 29459 regulates the registration and supervision of medical devices, which is particularly relevant for portable technology.
Why is informed consent important in clinical trials?
Informed consent is crucial because all participants must fully understand the study's purpose, risks, and benefits before participating.
What should researchers know about information protection regulations?
Researchers should be familiar with information protection regulations to secure participant details, especially when using wearable devices that collect sensitive health information.
How does bioaccess® ensure information security?
bioaccess® is committed to maintaining information security and client confidence, addressing any issues through established grievance and data protection protocols.
What role does the INS's DIIS play in clinical studies?
The INS's DIIS supervises the ethical review process for clinical studies, emphasizing the importance of ethical adherence in study design.
Why is it important to understand the fee structures related to Institutional Ethics Committee evaluations?
Understanding the fee structures can provide valuable insights into potential expenses and fluctuations in the ethics review process, which is crucial for designing trials for wearables in Peru.
Where can researchers find comprehensive information on the regulatory environment in Peru?
Researchers can refer to resources such as the ClinRegs database and the official INS website for comprehensive information on the regulatory environment.
How can bioaccess® assist in the clinical study process?
bioaccess® specializes in Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and Post-Market Clinical Follow-Up Studies (PMCF), helping to streamline clinical study processes and ensure a successful transition from pilot study to commercialization.