


This article emphasizes the critical importance of engaging clinical study participants by implementing proven best practices such as clear communication, informed consent, and the incorporation of participant feedback. These strategies are not just beneficial; they are essential for enhancing understanding and trust among participants. As a result, this leads to improved recruitment and retention in clinical trials. The necessity for transparency and tailored communication approaches to diverse populations is underscored, highlighting the relevance of these practices in the clinical research landscape.
Engaging participants in clinical studies is a pivotal aspect of advancing medical research. However, many struggle to understand the intricacies of this process. With only a fraction of individuals fully grasping the phases and significance of their involvement, the need for effective communication and tailored strategies becomes evident.
This article delves into best practices for enhancing participant engagement, including:
Ultimately, it raises the question: how can researchers transform the clinical trial experience to empower and retain participants effectively?
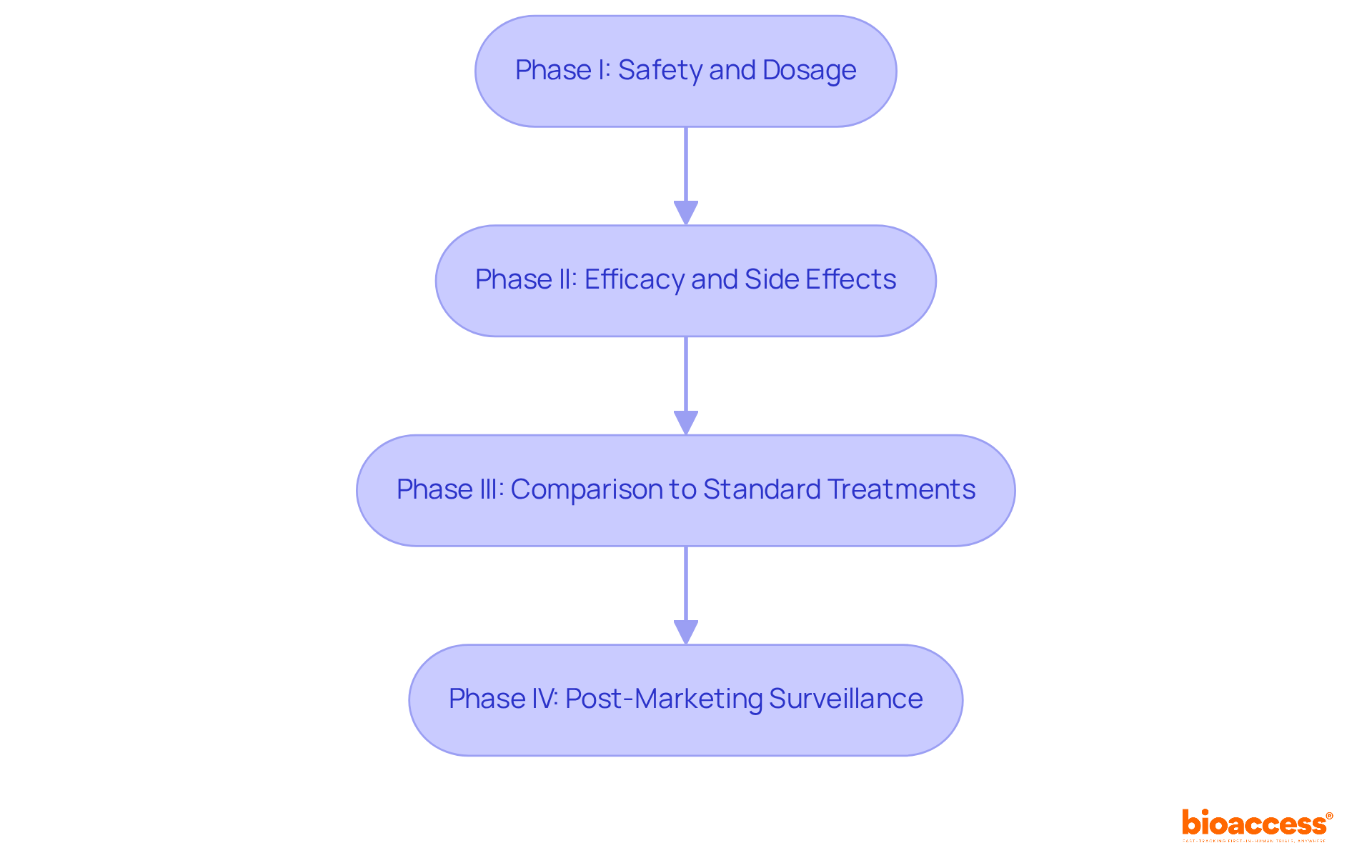
Clinical experiments represent meticulously organized research studies designed to evaluate the safety and effectiveness of new medical interventions. These studies progress through four distinct phases:
Understanding these phases is crucial for all participants, as it clarifies the purpose and potential impact of their involvement, fostering a sense of contribution to medical advancements. For instance, individuals participating in Phase I studies are instrumental in determining safe dosage levels, which are vital for the success of subsequent phases.
Notably, studies indicate that only 47% of individuals fully comprehend the stages of clinical studies, underscoring the need for effective communication strategies. Implementing clear and accessible information regarding each stage and its significance can significantly enhance retention and satisfaction during the process. By equipping participants with essential information, clinical trial organizations can improve recruitment outcomes and ensure that studies are conducted effectively and ethically.
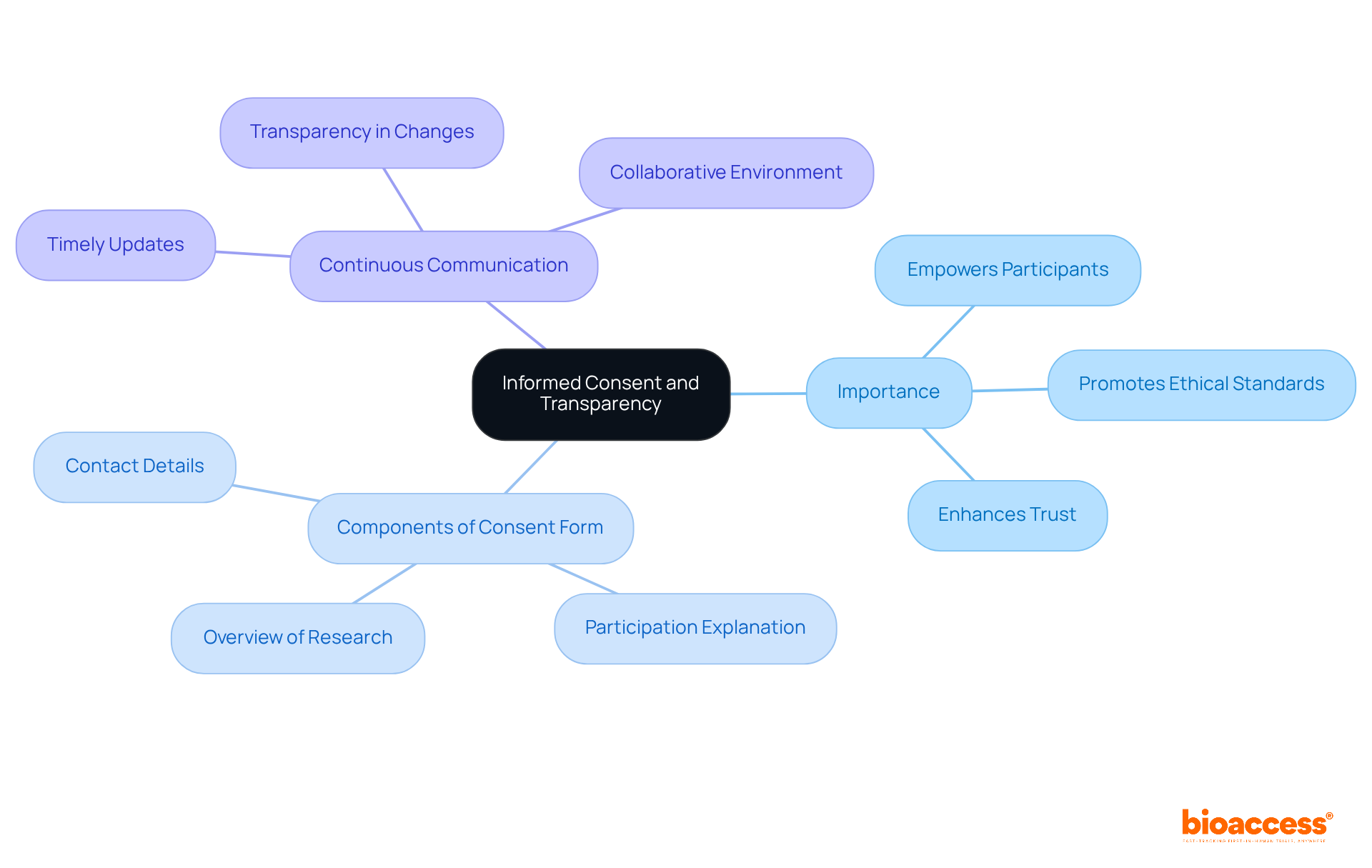
Informed consent is paramount in clinical research; it must be clear, comprehensive, and accessible, thereby empowering participants to make informed decisions about their involvement. Researchers are tasked with providing detailed information regarding the research's purpose, procedures, potential risks, and benefits. A well-organized consent form should include:
Furthermore, maintaining continuous communication throughout the trial is essential; individuals should receive timely updates on any changes to the research or new findings that may influence their decision to continue. This level of transparency not only respects individuals' autonomy but also cultivates a collaborative environment that significantly enhances participation and trust.
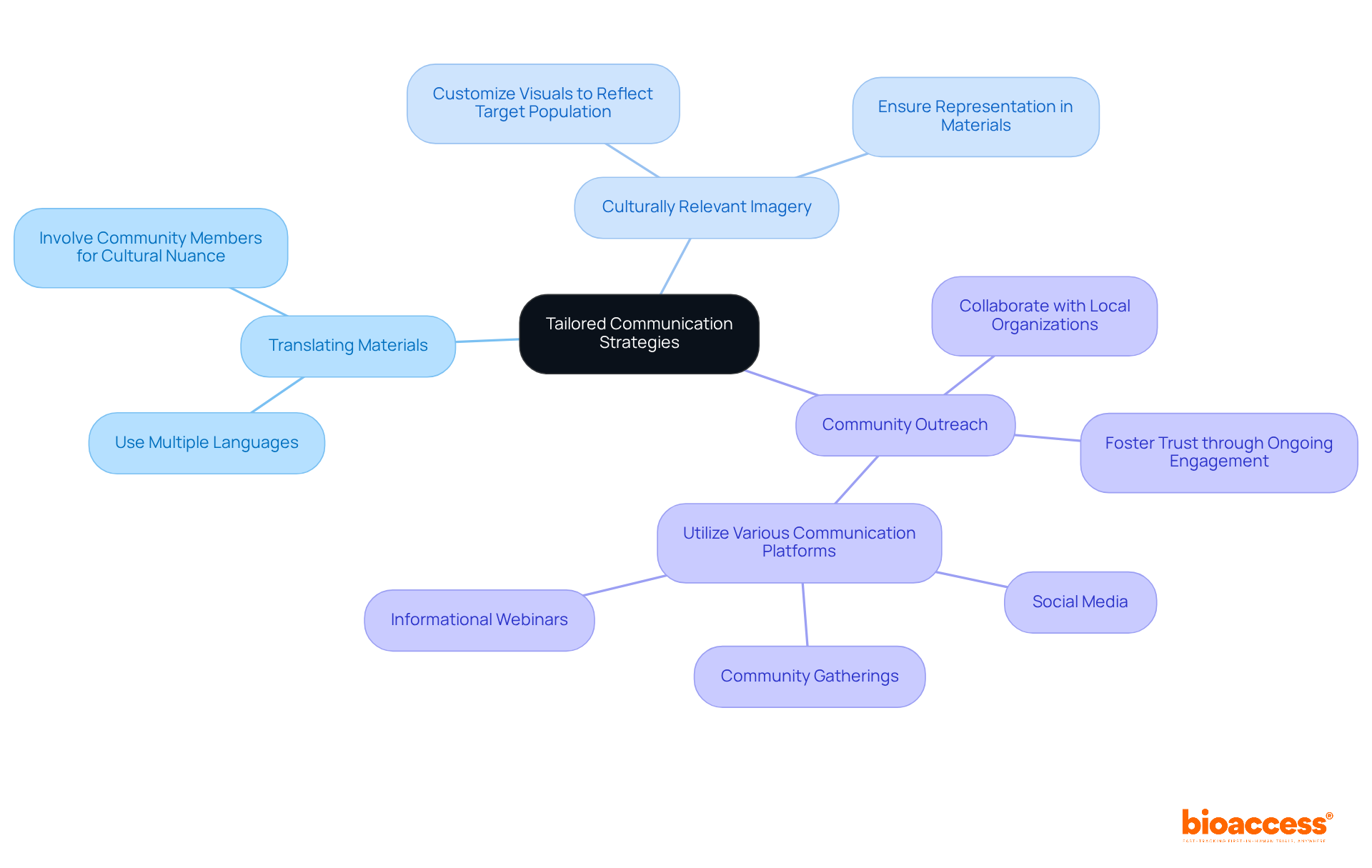
To effectively engage diverse populations, researchers must develop communication strategies that recognize cultural, linguistic, and educational differences. This involves:
For instance, collaborating with local organizations can enhance access to underrepresented groups, enabling researchers to deliver tailored information that addresses specific needs and concerns. Furthermore, utilizing various communication platforms—such as social media, community gatherings, and informational webinars—can significantly bolster outreach efforts, ensuring that potential attendees and clinical study participants are well-informed and empowered to make decisions regarding their participation.
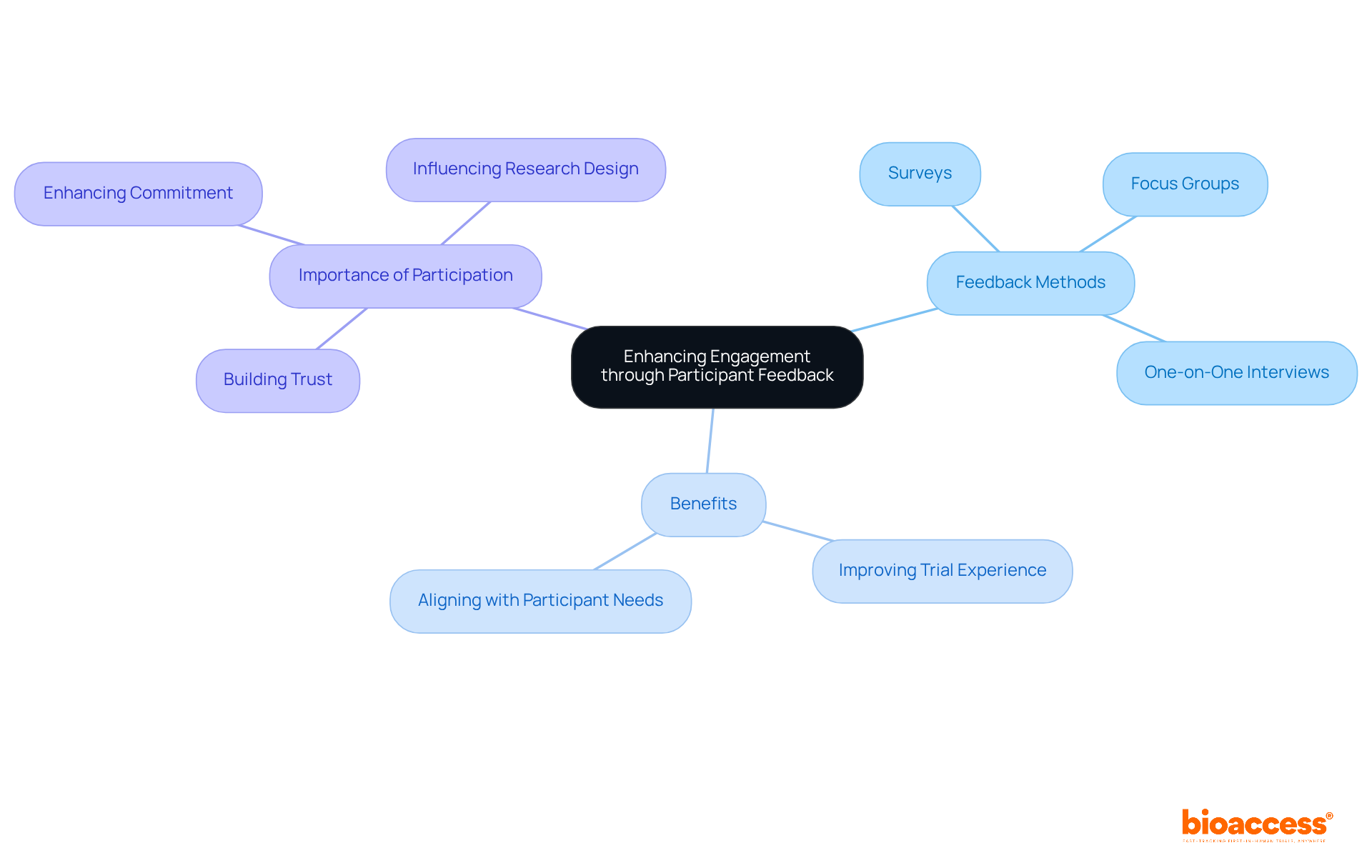
Actively seeking and addressing feedback from clinical study participants is essential for enhancing engagement and satisfaction in clinical trials. Researchers must implement various mechanisms, such as:
to facilitate open communication regarding the experiences of clinical study participants. Collecting feedback on research procedures can unveil opportunities for improvement, such as reducing visit frequency or simplifying consent forms. The Clinical Trials Transformation Initiative (CTTI) underscores that active participation of clinical study participants is crucial for the overall success of clinical studies. Furthermore, transparently communicating how feedback from contributors has influenced research design strengthens their sense of value and commitment to the research process. Utilizing digital tools and telemedicine platforms can further enhance the feedback process, making it more accessible for individuals involved. This iterative approach not only improves the trial experience but also significantly contributes to the overall success of the study by aligning it more closely with the needs and preferences of those involved. As Alicia Staley, senior director of patient engagement, asserts, "If our patient advocates haven’t looked at something rolling out the door, an explanation is expected as to why not." This statement highlights the critical importance of incorporating the insights of clinical study participants into the research process.
Engaging clinical study participants is pivotal for the success of medical research, necessitating a comprehensive understanding of best practices that enhance their experience and involvement. By focusing on informed consent, transparent communication, tailored strategies for diverse populations, and actively incorporating participant feedback, researchers can create a more inclusive and effective research environment. This multifaceted approach not only respects the autonomy of participants but also fosters a collaborative atmosphere that can lead to more meaningful outcomes in clinical trials.
The article highlights the importance of clearly communicating the phases of clinical trials, ensuring participants are well-informed about what to expect. It emphasizes that informed consent should be thorough and accessible, empowering individuals to make educated decisions about their participation. Additionally, the necessity of culturally sensitive communication strategies is underscored, as these practices can significantly improve engagement with underrepresented populations. Finally, the integration of participant feedback is presented as a vital tool for enhancing the trial experience and aligning research with the needs of those involved.
As the landscape of clinical research continues to evolve, it is crucial for stakeholders to prioritize these best practices. By doing so, they can not only improve participant retention and satisfaction but also contribute to the advancement of medical knowledge and innovation. Embracing these strategies will ultimately lead to more successful clinical trials and better health outcomes for the communities they serve.
What are clinical trials?
Clinical trials are meticulously organized research studies designed to evaluate the safety and effectiveness of new medical interventions.
What are the four phases of clinical trials?
The four phases of clinical trials are: - Phase I: Focuses on safety and dosage. - Phase II: Assesses efficacy and side effects. - Phase III: Compares the new intervention to standard treatments. - Phase IV: Involves post-marketing surveillance.
Why is it important for participants to understand the phases of clinical trials?
Understanding the phases is crucial for participants as it clarifies the purpose and potential impact of their involvement, fostering a sense of contribution to medical advancements.
What role do participants play in Phase I clinical trials?
Participants in Phase I studies are instrumental in determining safe dosage levels, which are vital for the success of subsequent phases.
What percentage of individuals comprehend the stages of clinical studies?
Studies indicate that only 47% of individuals fully comprehend the stages of clinical studies.
How can communication strategies improve participants' experience in clinical trials?
Implementing clear and accessible information regarding each stage and its significance can enhance retention and satisfaction, improve recruitment outcomes, and ensure studies are conducted effectively and ethically.