


This article emphasizes the enhancement of scientific validity in clinical research practices by establishing core principles, including internal, external, and construct validity. It advocates for the implementation of rigorous study design methodologies and strict ethical compliance. By detailing strategies such as randomization, blinding, and effective recruitment techniques, it reinforces the notion that adherence to these principles and methodologies results in more reliable and impactful research outcomes.
In the intricate realm of clinical research, the pursuit of scientific validity serves as a fundamental pillar for generating reliable and impactful findings. As the demand for credible evidence escalates, researchers face the challenge of navigating a multitude of principles and methodologies that not only bolster the integrity of their studies but also cultivate trust among stakeholders.
However, with trials growing increasingly complex and the urgent need for ethical compliance, how can researchers effectively balance rigorous design with the hurdles of participant recruitment and regulatory adherence?
This article explores essential strategies for enhancing scientific validity in clinical research, providing insights that have the potential to reshape the landscape of medical investigation.
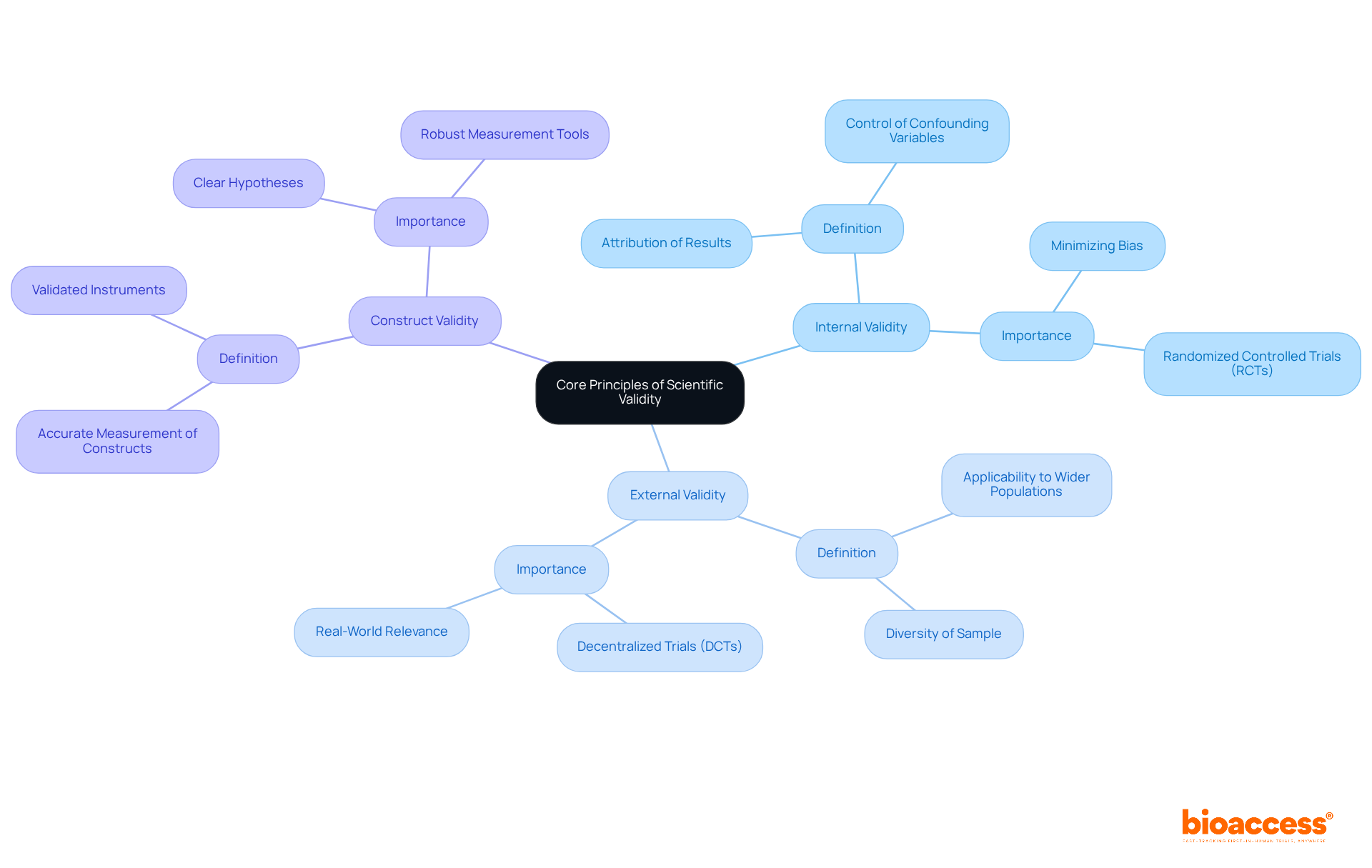
Researchers must establish core principles that direct their investigations to enhance scientific validity. These principles include:
Internal Validity: This refers to the extent to which the results of an investigation can be attributed to the interventions tested rather than other factors. Researchers should rigorously control for confounding variables and create experiments that minimize bias. For instance, randomized controlled trials (RCTs) are often lauded for their ability to provide unbiased estimates of treatment effects; yet, they require careful execution to ensure that randomization effectively balances known and unknown confounders. bioaccess's compliance reviews can help ensure that these standards are met.
External Validity: This principle evaluates the applicability of findings to wider populations. Researchers must consider the diversity of their sample and the settings in which the study is conducted. For instance, decentralized trials (DCTs) have arisen as a promising method to improve external validity by fostering inclusivity and tackling logistical obstacles that frequently restrict participant diversity in conventional RCTs. This adaptability guarantees that outcomes are relevant in real-world situations, which is becoming increasingly crucial in 2025 as the need for pertinent evidence rises. bioaccess's project management services can facilitate the effective implementation of DCTs.
Construct Validity: This involves ensuring that the study accurately measures the theoretical constructs it claims to measure. Researchers should employ validated instruments and methodologies to assess outcomes effectively. The importance of construct validity is underscored by the need for clear hypotheses and robust measurement tools, which are essential for drawing meaningful conclusions from clinical research. bioaccess's trial setup services can assist in establishing these robust methodologies.
By adhering to these principles, researchers can greatly enhance the scientific validity of their investigations, resulting in more reliable and influential outcomes. This dedication to strict standards not only propels the area of medical research but also cultivates increased trust among stakeholders in the results generated. Moreover, bioaccess's extensive trial management services—including feasibility assessments, site selection, compliance evaluations, trial preparation, import permits, project oversight, and reporting—play a vital role in assisting researchers in upholding these standards. Furthermore, the influence of Medtech research initiatives extends beyond investigation, aiding local economies through job creation, economic development, and healthcare enhancement, thus promoting international cooperation. Researchers must remain aware of the inherent uncertainties concerning risks and benefits in medical research, as these elements play a crucial role in the overall validity of research outcomes.
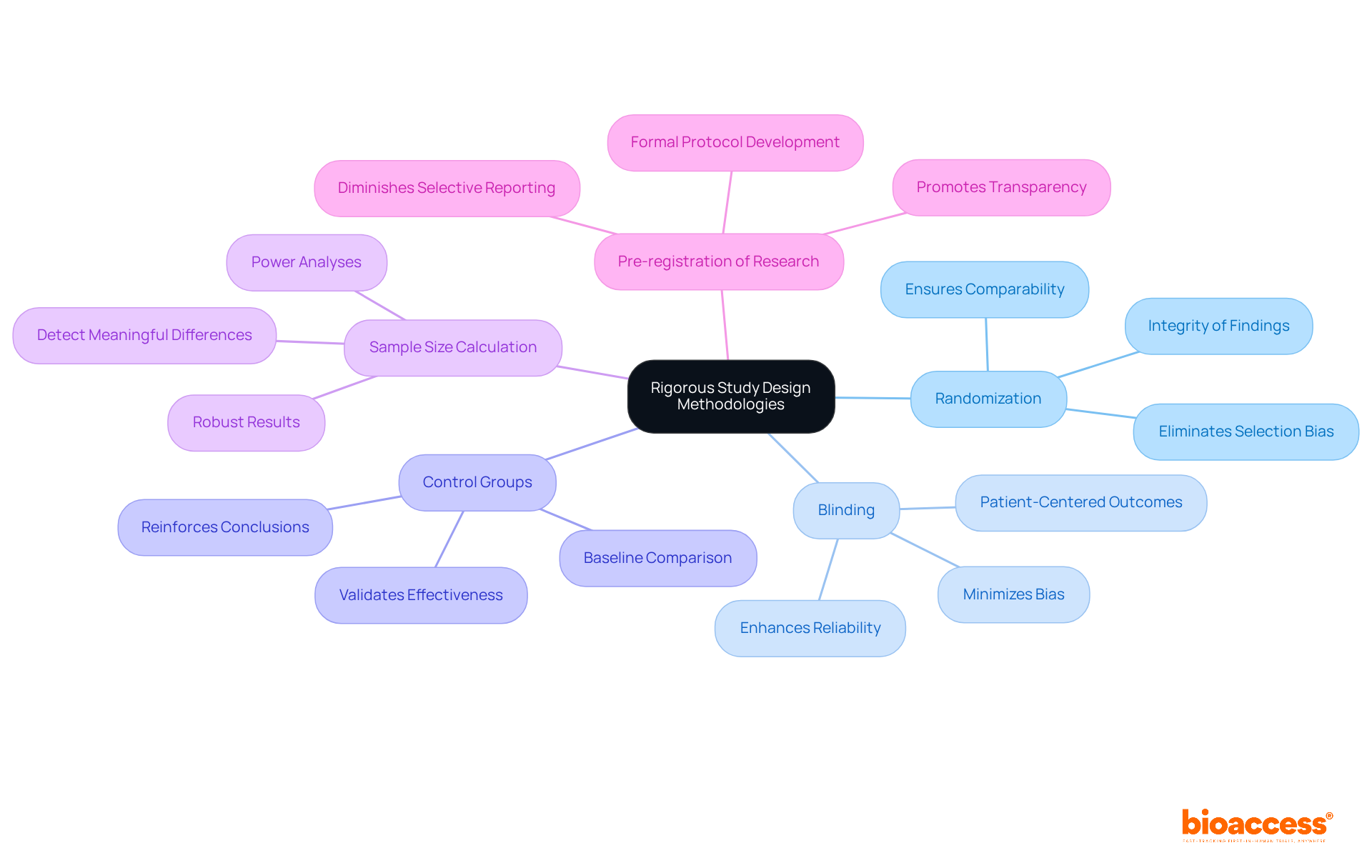
Implementing rigorous research design methodologies is essential to ensure the scientific validity of clinical research. Key strategies include:
Randomization: Randomly assigning participants to treatment and control groups eliminates selection bias, ensuring comparability between groups. This method is essential for preserving the integrity of the research's findings. As emphasized in the case analysis on adaptive trial designs, proper randomization is essential for obtaining trustworthy results.
Blinding: Employing single or double-blind designs minimizes bias in outcome assessment. When participants and/or researchers are unaware of group assignments, it prevents expectations from influencing results, thereby enhancing the reliability of the data collected. The significance of blinding is highlighted by the Patient-Centered Outcomes Research Institute (PCORI), which stresses that patient-centered results should be the emphasis of examinations on medical tests.
Control Groups: The inclusion of a control group allows for comparison against a baseline, which is vital for validating the effectiveness of the intervention being studied. This method reinforces the overall conclusions derived from the research, as highlighted in the guidelines for evaluations of medical tests, which emphasize the necessity for clear comparators.
Sample Size Calculation: Conducting power analyses to determine the appropriate sample size is critical. Adequate sample sizes ensure that the research possesses sufficient power to detect meaningful differences, thereby enhancing the robustness of the results. The criteria for adaptive and Bayesian trial designs emphasize the importance of detailing sample size calculations in the protocol.
Pre-registration of Research: Pre-registering research and their methodologies promotes transparency and diminishes the likelihood of selective reporting. This practice is increasingly acknowledged as a benchmark for improving the credibility of medical research. As stated in the guidelines, researchers should develop a formal protocol that provides the plan for conducting the research.
By adopting these rigorous methodologies, researchers can significantly enhance the scientific validity and quality of their research, ultimately contributing to more reliable and actionable results in the field. However, it is essential to be aware of common pitfalls, such as misapplying randomization or blinding, which can undermine the study's integrity. Addressing these issues proactively can further enhance the credibility of medical research.
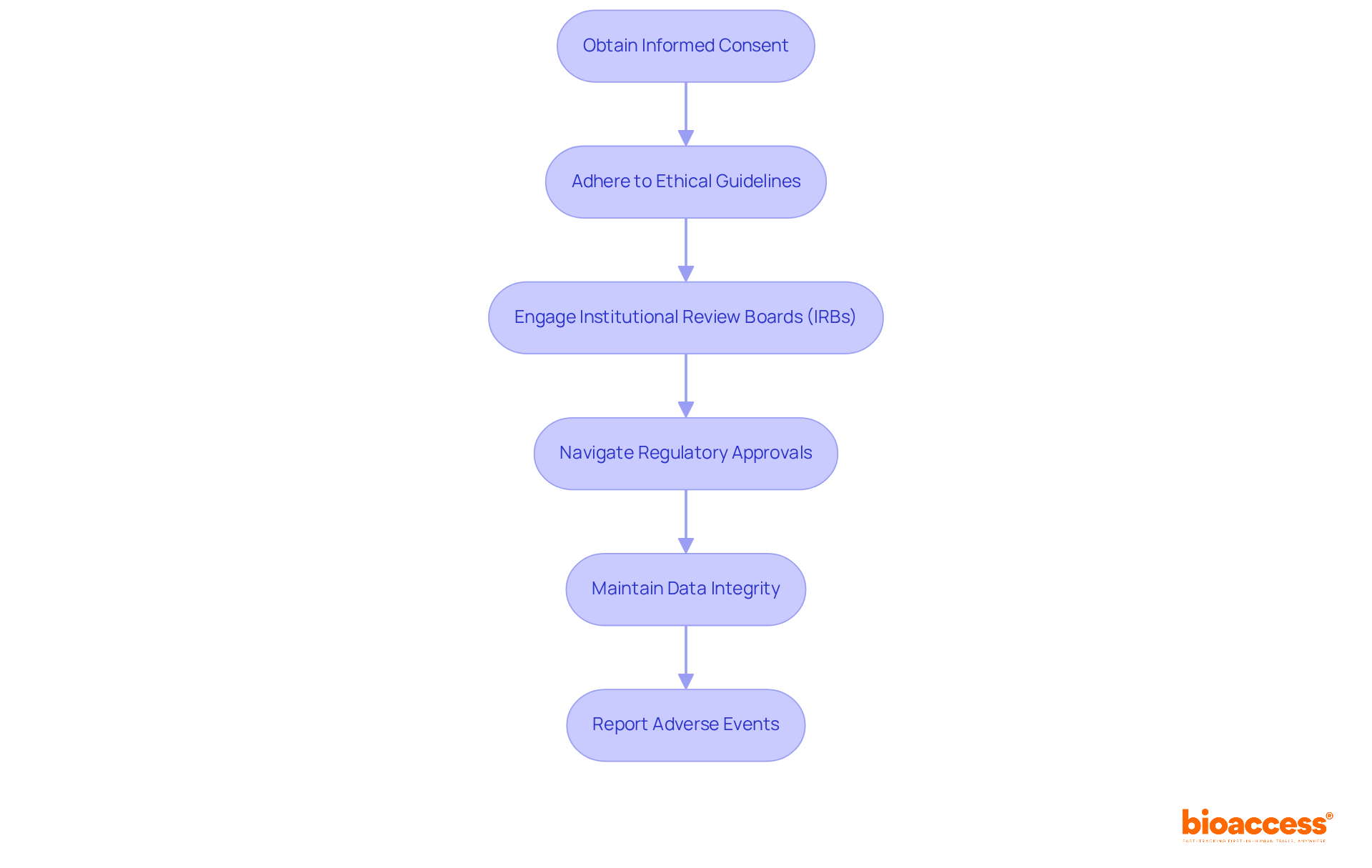
Ethical compliance and regulatory adherence stand as essential pillars of clinical research. Researchers must prioritize several critical actions:
Obtain Informed Consent: It is crucial that all individuals provide informed consent, fully understanding the risks and benefits associated with their involvement in the study. This process empowers individuals to make informed choices regarding their participation.
Adhere to Ethical Guidelines: Following established ethical frameworks, such as the Declaration of Helsinki, is vital. These guidelines outline essential principles for conducting research involving human subjects, ensuring respect for individuals' rights and welfare.
Engage Institutional Review Boards (IRBs): Submitting research protocols to IRBs for thorough review and approval is necessary to confirm that projects align with ethical standards and adequately protect participant rights. In Colombia, this involves obtaining approval for research from the site's IRB/ethics committee, which is a critical step in the regulatory process.
Navigate Regulatory Approvals: After IRB approval, researchers must secure project approval from Colombia's regulatory agency, INVIMA, and obtain an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational devices. This multi-step process can present challenges, particularly for medical device startups facing regulatory hurdles and competition.
Maintain Data Integrity: Implementing robust data management practices is essential for ensuring the accuracy, confidentiality, and reliability of user data. Secure electronic data capture systems and regular quality checks are necessary to uphold data integrity.
Report Adverse Events: Establishing clear protocols for the prompt reporting of adverse events to regulatory bodies is critical. This practice not only guarantees participant safety but also upholds adherence to regulatory requirements, including ongoing reporting of trial status and any significant or minor adverse events to INVIMA.
By prioritizing ethical compliance and regulatory adherence, researchers can cultivate trust and credibility in their work, ultimately enhancing the scientific validity of their findings. Furthermore, bioaccess provides extensive management services for research projects, encompassing feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, to assist researchers in navigating these intricate processes.
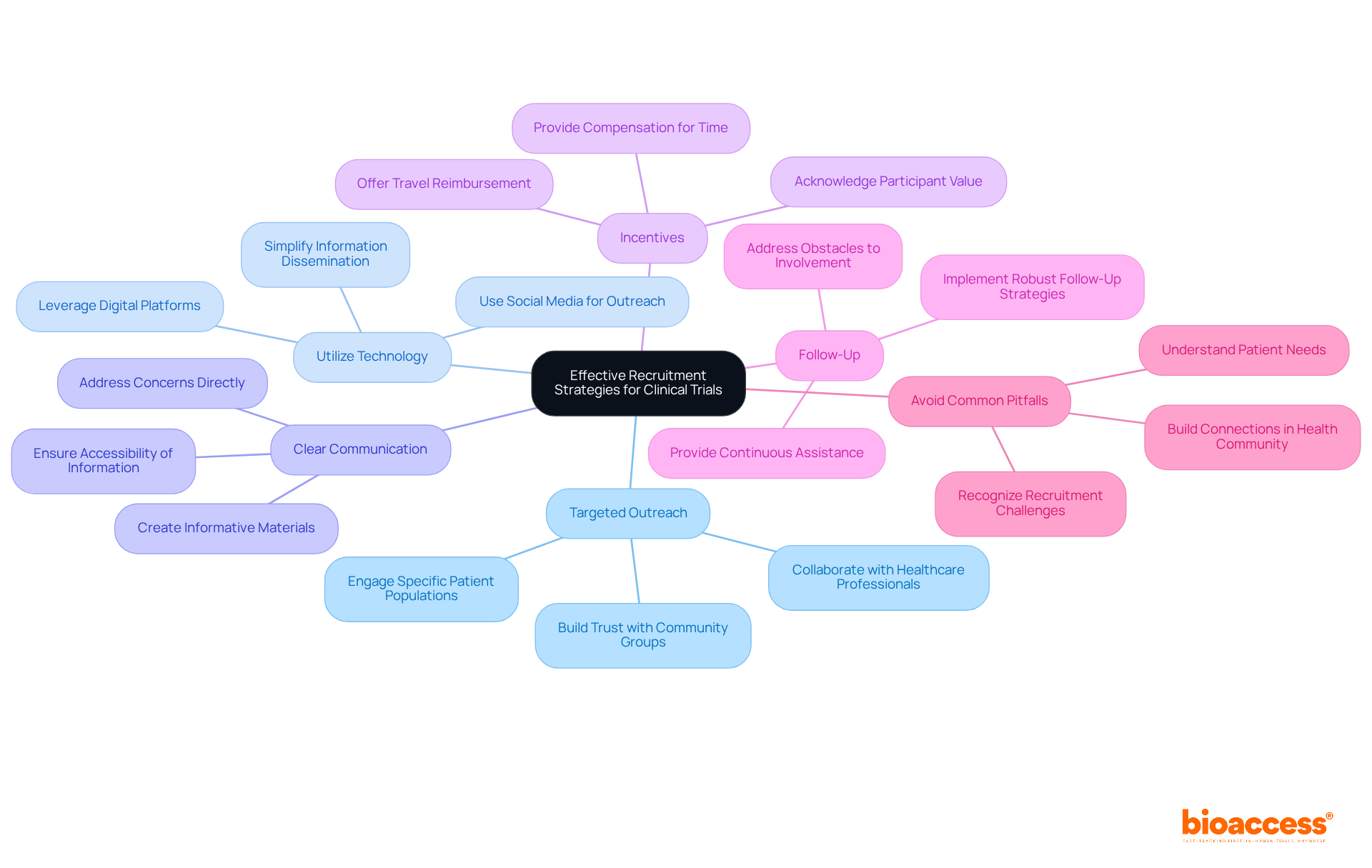
Effective recruitment strategies are crucial for the success of clinical trials. Researchers should adopt the following approaches:
Targeted Outreach: Engage specific patient populations that meet research criteria through focused outreach efforts. Collaborating with healthcare professionals and community groups enhances credibility and trust, fostering better connections with potential enrollees.
Utilize Technology: Harness digital platforms and social media to reach a broader audience. These tools can efficiently disseminate information regarding the research and its advantages, simplifying the process for prospective individuals to understand their engagement.
Clear Communication: Recruitment materials must be clear, informative, and accessible. Directly addressing potential individuals' concerns and questions can significantly enhance their willingness to engage in the study.
Incentives: Offering incentives, such as reimbursement for travel expenses or compensation for time, can motivate participation. This approach acknowledges the value of contributors' input and can improve enrollment rates.
Follow-Up: Implementing robust follow-up strategies is essential to maintain engagement with potential participants. Addressing obstacles to involvement and providing continuous assistance can help ensure that interested individuals remain informed and motivated to participate in the study.
Avoid Common Pitfalls: Researchers should be aware of common pitfalls in recruitment, such as failing to understand patient needs or neglecting to build connections within the health community. Addressing these challenges is vital for successful recruitment.
A notable example of effective recruitment strategies in action is the partnership between GlobalCare Clinical Trials and bioaccess™. This collaboration has led to a significant increase in ambulatory services for research in Colombia, resulting in more than a 50% decrease in recruitment duration and an impressive retention rate exceeding 95%. By creating and executing these effective recruitment strategies, researchers can boost participant enrollment and retention, ultimately enhancing the scientific validity and overall impact of their studies. Notably, 85% of all clinical trials fail to recruit sufficient patients, underscoring the necessity of these strategies.
Enhancing scientific validity in clinical research is not merely a best practice; it is a foundational necessity for producing reliable and impactful results. By concentrating on internal, external, and construct validity, researchers can ensure that their findings are not only accurate but also applicable to broader populations. This unwavering commitment to scientific rigor fosters trust among stakeholders and significantly contributes to the advancement of medical knowledge.
The article delineates several key strategies for achieving this validity:
Ultimately, the significance of scientific validity in clinical research extends beyond the confines of individual studies. It influences healthcare outcomes, drives economic development, and fosters international collaboration. Researchers are encouraged to adopt these best practices and remain vigilant in their pursuit of scientific excellence, as the future of medical research hinges on their steadfast commitment to upholding these standards.
What are the core principles of scientific validity that researchers should establish?
The core principles of scientific validity include internal validity, external validity, and construct validity.
What is internal validity?
Internal validity refers to the extent to which the results of an investigation can be attributed to the interventions tested rather than other factors. It requires rigorous control of confounding variables and minimizing bias, often achieved through randomized controlled trials (RCTs).
How can researchers enhance internal validity?
Researchers can enhance internal validity by carefully executing randomized controlled trials to ensure effective randomization and by controlling for confounding variables.
What is external validity?
External validity evaluates the applicability of findings to wider populations. It considers the diversity of the sample and the settings in which the study is conducted.
How can decentralized trials (DCTs) improve external validity?
Decentralized trials can improve external validity by fostering inclusivity and addressing logistical challenges that limit participant diversity in traditional RCTs, ensuring that outcomes are relevant in real-world situations.
What is construct validity?
Construct validity involves ensuring that the study accurately measures the theoretical constructs it claims to measure. It requires the use of validated instruments and methodologies to assess outcomes effectively.
Why is construct validity important?
Construct validity is important because it ensures that researchers have clear hypotheses and robust measurement tools, which are essential for drawing meaningful conclusions from clinical research.
How can bioaccess assist researchers in maintaining scientific validity?
Bioaccess offers various trial management services, including compliance reviews, project management for decentralized trials, and trial setup services, which help researchers uphold scientific validity standards.
What are the broader impacts of Medtech research initiatives?
Medtech research initiatives contribute to local economies through job creation, economic development, and healthcare enhancement, while also promoting international cooperation.
What should researchers be aware of regarding risks and benefits in medical research?
Researchers must remain aware of the inherent uncertainties concerning risks and benefits in medical research, as these elements significantly impact the overall validity of research outcomes.