

Excipients are inactive substances in pharmaceuticals that serve critical functions in drug formulation. They enhance stability, facilitate manufacturing, and improve patient compliance. This article underscores their significance by illustrating how excipients, such as binders and fillers, ensure the effective delivery of active ingredients. Furthermore, it highlights the increasing market demand for these essential components within the pharmaceutical industry.
Excipients play a crucial yet often overlooked role in the pharmaceutical landscape, serving as the unsung heroes of medication formulations. These inactive ingredients are not merely fillers; they enhance drug stability, facilitate manufacturing processes, and improve patient compliance, ensuring that active pharmaceutical ingredients (APIs) perform effectively.
However, the selection of excipients presents significant challenges, raising questions about their compatibility, safety, and impact on drug efficacy. Understanding the intricate dynamics of excipients is essential for anyone seeking to grasp their significance in the realm of pharmaceuticals.
The excipient definition states that excipients are integral components of pharmaceutical formulations, distinct from the active pharmaceutical ingredient (API). Their inclusion is essential for various functions, such as:
While these inactive ingredients do not exert therapeutic effects independently, their excipient definition illustrates their pivotal role in ensuring the overall efficacy and safety of medications. For example, excipients serve as:
This guarantees that the active ingredients are delivered effectively and safely to patients.
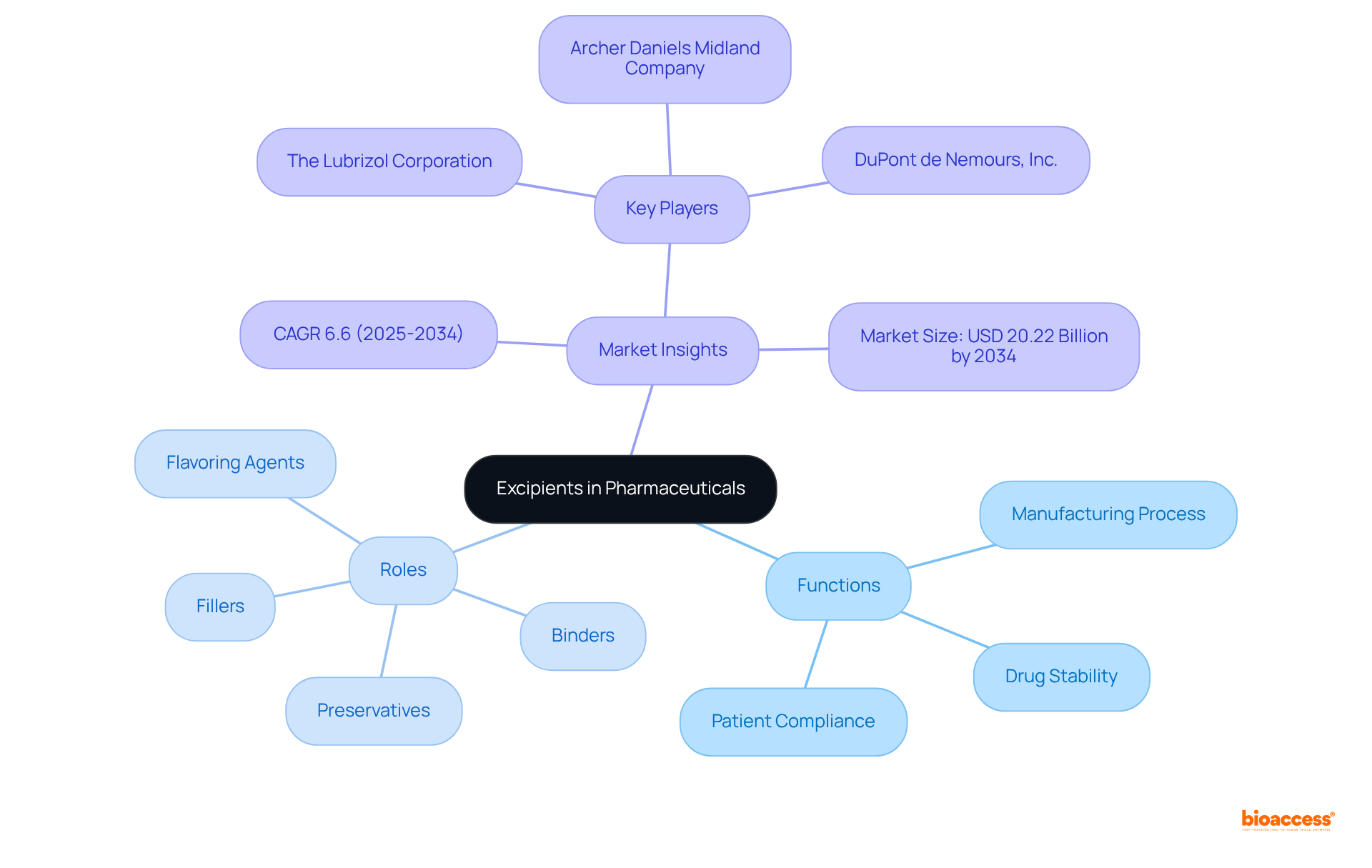
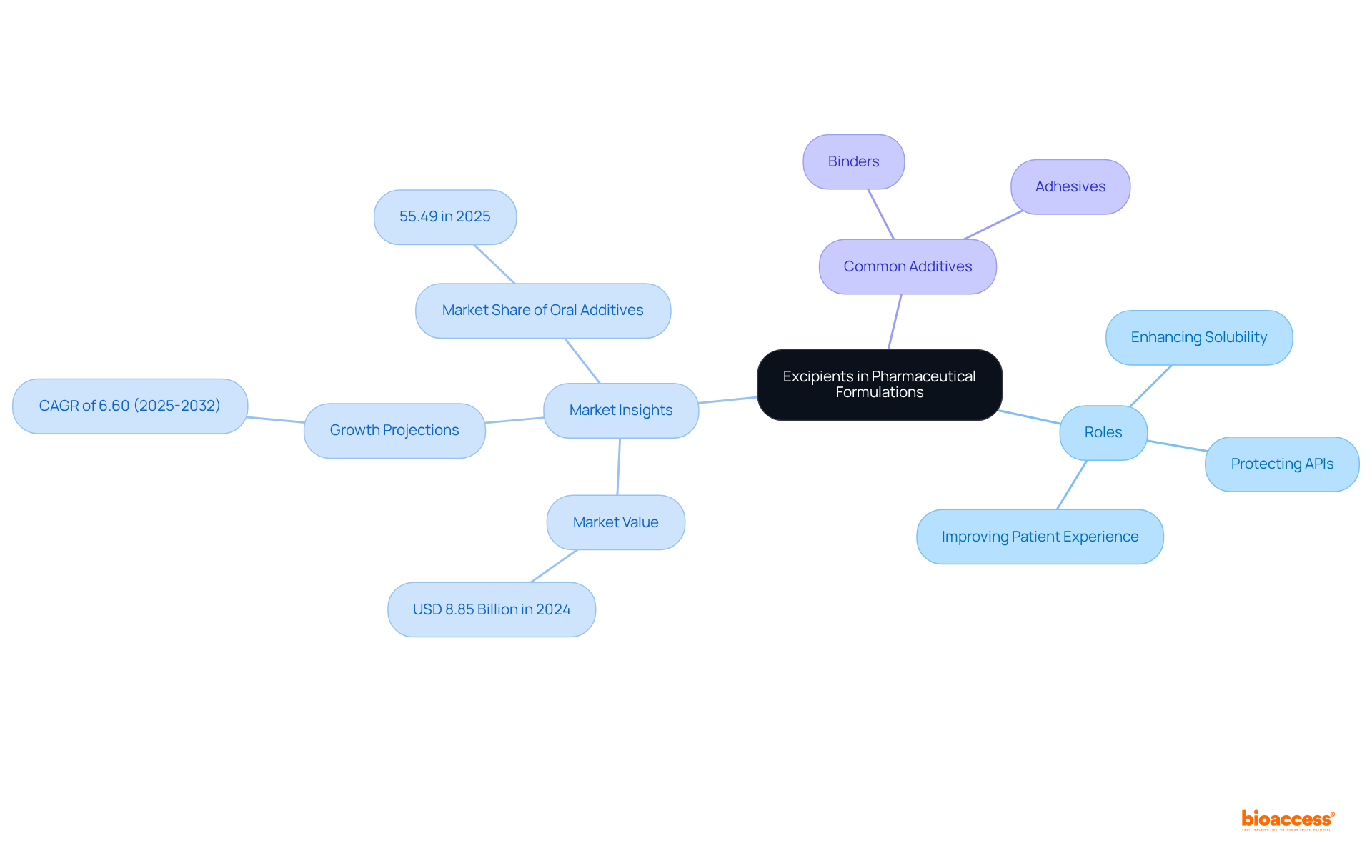
The excipient definition highlights that excipients are pivotal components in medicinal formulations, playing a significant role in drug delivery systems. They enhance the solubility and bioavailability of active medicinal ingredients (APIs), protect them from degradation, and streamline the manufacturing process. For instance, in solid dosage forms such as tablets, substances like microcrystalline cellulose act as fillers, providing bulk and ensuring consistent dosing. The global pharmaceutical additives market was valued at approximately USD 8.85 billion in 2024, with projections indicating a growth rate of 6.60% from 2025 to 2032, underscoring the increasing demand for these essential substances.
Moreover, the excipient definition indicates that inactive ingredients are vital in enriching the overall patient experience by improving the flavor and appearance of medications, which holds particular importance in pediatric formulations. The oral additives segment is expected to dominate the market, representing a considerable share due to their ease of administration and high patient adherence. Experts assert that the careful selection of additives can significantly enhance the bioavailability of medications, rendering them more effective in therapeutic applications. Frequently utilized additives in solid dosage forms include:
These are projected to command the largest market share of 56.72% in 2023, emphasizing their critical role in maintaining the structural integrity and uniformity of drug content. Furthermore, the oral additives segment is anticipated to account for a market share of 55.49% in 2025, further illustrating the importance of additives in drug formulations.
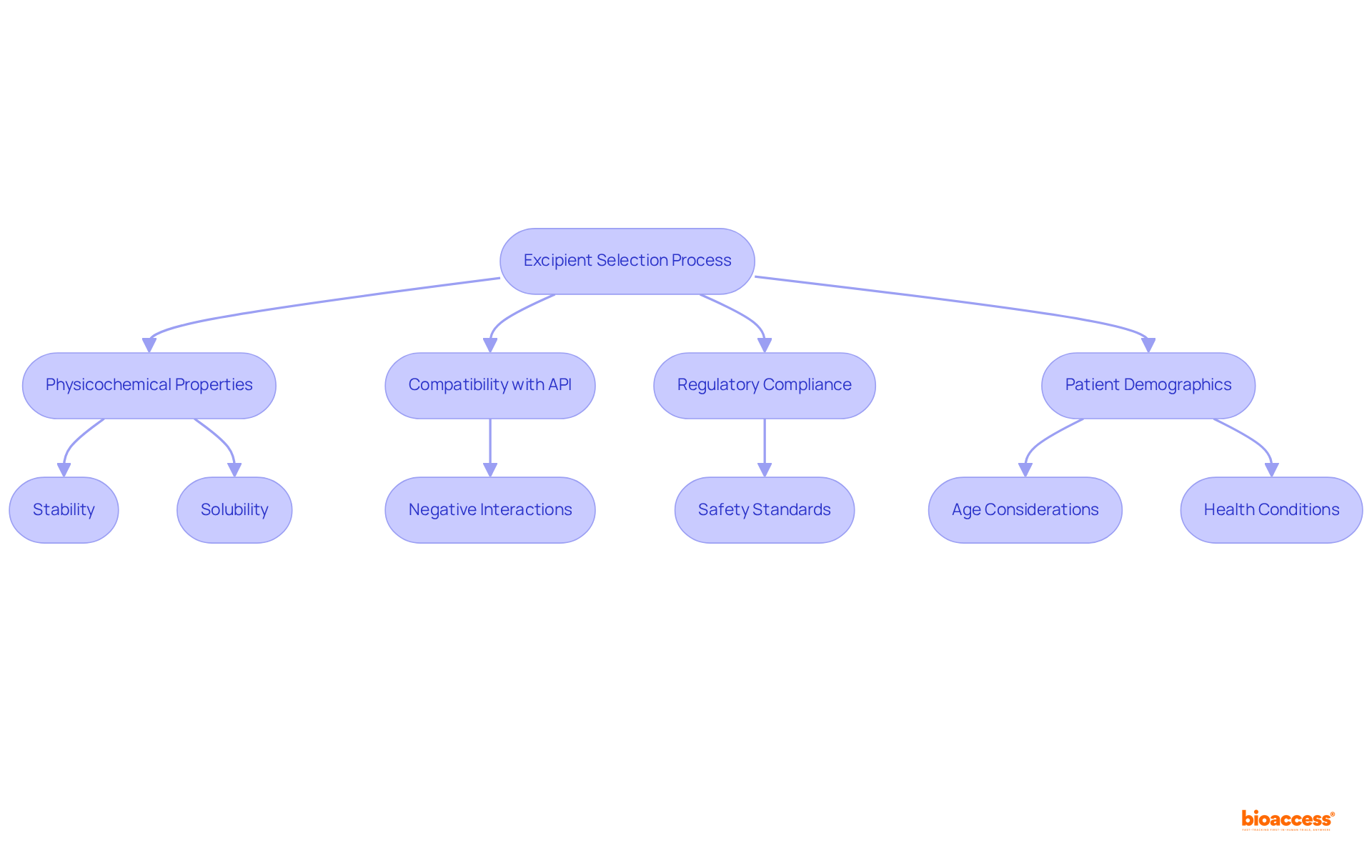
Choosing suitable additives is a multifaceted process that hinges on several essential considerations. The physicochemical properties of the active pharmaceutical ingredient (API) are pivotal, influencing stability, solubility, and bioavailability. Inactive substances must demonstrate compatibility with the API to prevent negative interactions that could undermine drug effectiveness. Understanding the relationship between the chemical structure of additives and their functional effects is crucial in this context. Moreover, the intended route of administration—be it oral, injectable, or topical—determines specific characteristics according to the excipient definition, such as viscosity and particle size, which are vital for achieving the desired release profile.
Regulatory compliance emerges as another critical factor in excipient selection. The excipient definition highlights that excipients must adhere to stringent safety and quality standards, as they can constitute 80-90% of the final medicinal product. Recent statistics reveal that the global pharmaceutical excipients market was valued at USD 8.85 billion in 2024, with projections to reach USD 14.77 billion by 2032, reflecting a CAGR of 6.60% from 2025 to 2032. This underscores the increasing significance of excipient quality in drug development.
Furthermore, patient demographics, including age and health conditions, should inform the selection process to ensure that products are both safe and effective for their intended populations. Best practices for selecting additives in 2025 stress the importance of a thorough understanding of the physicochemical properties of these substances, coupled with a comprehensive evaluation of their functionality and potential impact on the overall formulation. Notably, the cost of regulatory compliance for additive manufacturers has surged by over 30% in the past five years, emphasizing the necessity for careful selection of compliant substances. By adhering to these criteria, pharmaceutical developers can significantly enhance the stability, efficacy, and safety of their products.
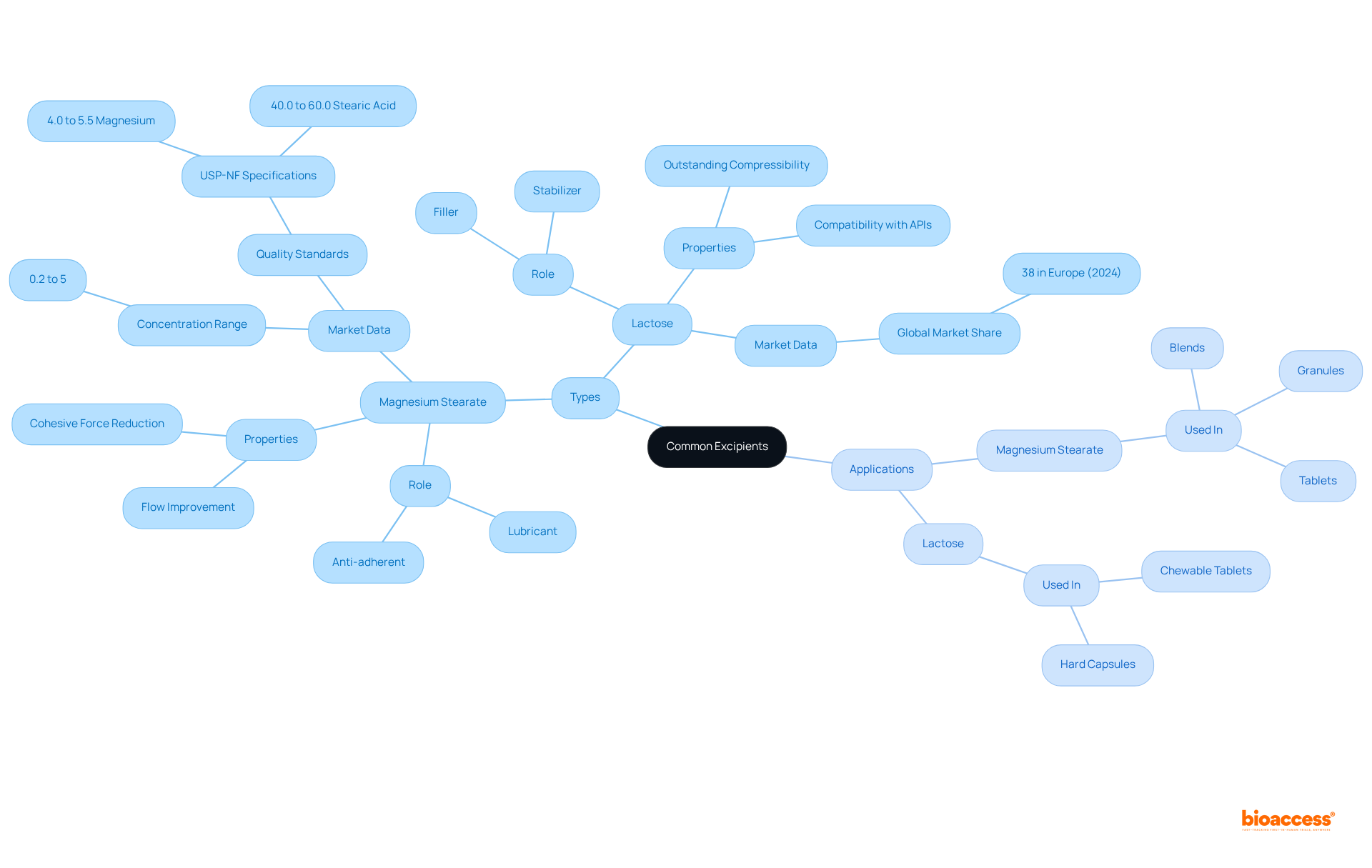
The excipient definition illustrates how common excipients play crucial roles in pharmaceutical formulations, each tailored to enhance the efficacy and manufacturability of medications. Magnesium stearate, a widely used lubricant, is essential for preventing ingredients from adhering to manufacturing equipment, thereby ensuring smooth production processes. Typically incorporated in concentrations ranging from 0.2% to 5%, it significantly improves the flow properties of drug powders by reducing cohesive forces between particles. This characteristic is vital for maintaining consistent quality and efficiency in pharmaceutical manufacturing. According to the USP-NF and EP monographs, magnesium stearate must contain specific percentages of magnesium and stearic acid, thereby guaranteeing its quality and efficacy in preparations.
Conversely, lactose serves primarily as a filler in tablet formulations, providing bulk and stability. It is especially preferred due to its outstanding compressibility and compatibility with various active medicinal ingredients (APIs). The global market for additives, which includes substances such as magnesium stearate and lactose, is projected to expand from USD 10.41 billion in 2024 to approximately USD 15.49 billion by 2034, indicating a compound annual growth rate (CAGR) of 4.06% from 2025 to 2034. Notably, Europe captured a significant market share of 38% in 2024, underscoring its dominance in the additives market.
The practical applications of these substances are evident in numerous pharmaceutical products. For instance, magnesium stearate is commonly found in blends, granules, and various tablet forms, ensuring that the manufacturing process remains efficient and that the final products meet quality standards. Lactose is also prevalent in chewable tablets and hard capsules, where it enhances the overall composition without compromising therapeutic efficacy. However, it is crucial to acknowledge that animal-derived magnesium stearate may raise concerns regarding contamination and sourcing, which formulators should carefully consider.
Understanding the excipient definition and the uses and significance of substances like magnesium stearate and lactose is essential for formulators aiming to create efficient, patient-friendly medications. As the demand for personalized medicines continues to rise, the role of these excipients becomes increasingly critical in ensuring that drug formulations are both stable and effective.
Excipients play an indispensable role in the realm of pharmaceuticals, serving as crucial components that enhance the effectiveness and safety of medications. While they may lack therapeutic properties on their own, their contributions to drug formulation—spanning from stability improvement to patient compliance facilitation—underscore their significance in the overall medicinal landscape.
This article explores various facets of excipients, from their definition and importance to the criteria for selecting the right additives. Key insights include the necessity of understanding the physicochemical properties of both excipients and active pharmaceutical ingredients (APIs), alongside the growing demand for high-quality excipients in the pharmaceutical market. The discussion highlights how excipients such as magnesium stearate and lactose are integral to ensuring consistent manufacturing processes and enhancing patient experience.
Given the critical functions that excipients serve, it is essential for pharmaceutical developers to prioritize their selection and application. As the industry evolves with a focus on personalized medicine and stringent regulatory standards, understanding the role of excipients becomes paramount. Emphasizing their importance not only in drug formulation but also in improving patient outcomes can lead to more effective and safer therapeutic options. Thus, a deeper awareness of excipients and their applications will empower stakeholders to innovate and optimize pharmaceutical products for the benefit of all.
What are excipients in pharmaceuticals?
Excipients are integral components of pharmaceutical formulations that are distinct from the active pharmaceutical ingredient (API). They play essential roles in the manufacturing process, enhancing drug stability, and promoting patient compliance.
Do excipients have therapeutic effects?
No, excipients do not exert therapeutic effects independently. However, they are crucial for ensuring the overall efficacy and safety of medications.
What functions do excipients serve in pharmaceutical formulations?
Excipients serve various functions, including acting as binders, fillers, preservatives, and flavoring agents, which help in delivering active ingredients effectively and safely to patients.
Why are excipients important in drug formulation?
Excipients are important because they facilitate the manufacturing process, enhance the stability of drugs, and promote patient compliance, ensuring that medications work effectively and safely.