


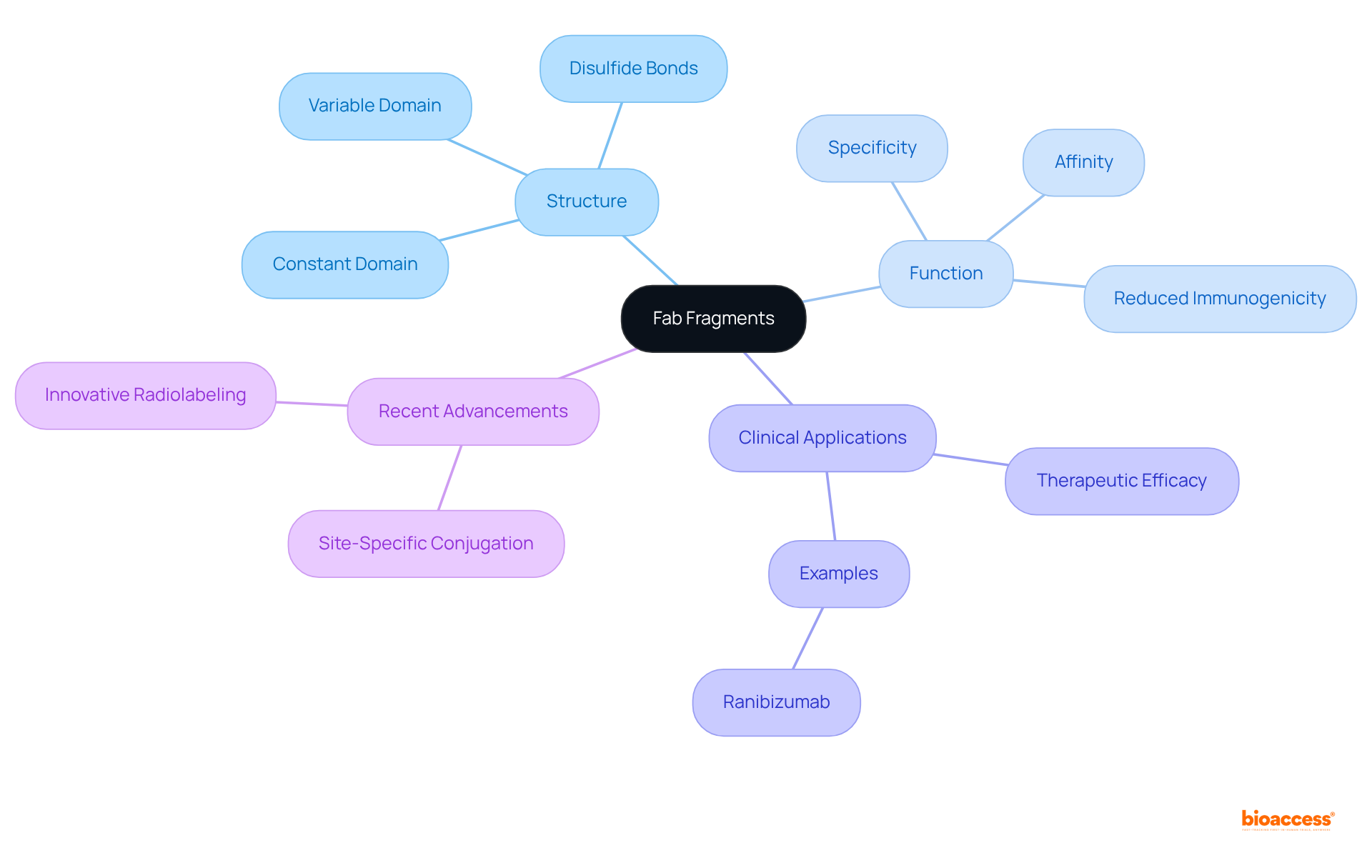
Fab fragments are defined as antigen-binding domains of immunoglobulins, comprising one constant and one variable domain from both heavy and light chains. This unique structure enables high specificity and affinity in binding to target antigens, while notably excluding the Fc region. The article underscores their advantages, such as:
These factors highlight the growing importance of Fab fragments in modern medicine, particularly within the realms of oncology and personalized treatment strategies.
Fab fragments have emerged as pivotal players in the landscape of immunology, fundamentally transforming the development and administration of targeted therapies. These antigen-binding domains, derived from antibodies, present unique advantages, including reduced immunogenicity and enhanced tissue penetration. Such characteristics position them as ideal candidates for a variety of medical applications.
As the field continues to advance, critical questions arise regarding the optimization of their effectiveness and the expansion of their use in clinical settings. What key characteristics contribute to the value of Fab fragments, and how can they be further leveraged to enhance patient outcomes in modern medicine?
Fab fragments, or antigen-binding domains, are essential components of immunoglobulins that retain the ability to bind to specific antigens. Each Fab portion comprises one constant and one variable domain derived from both the heavy and light chains of an antibody, interconnected by disulfide bonds. This distinctive structure enables Fab portions to exhibit high specificity and affinity for their target antigens while excluding the Fc region, which is responsible for mediating effector functions such as complement activation and binding to Fc receptors. The absence of the Fc region not only reduces immunogenicity but also enhances tissue penetration, rendering Fab portions particularly advantageous in medical applications.
Current research underscores the immunogenicity of the fab fragment components, showcasing their potential to mitigate adverse immune responses in clinical settings. For example, the development of Cu radiolabeled fab fragment components has demonstrated improved pharmacokinetics and therapeutic efficacy, as evidenced by studies utilizing these fragments for targeted imaging and treatment of tumors. Notably, ranibizumab, a fab fragment that was approved in 2006 for the treatment of wet age-related macular degeneration, exemplifies the clinical utility of these components in improving patient outcomes. As of April 2025, at least eight Fab antibody drugs have received global approval, underscoring their significance in modern medicine.
Recent advancements in therapeutic applications of fab fragment have focused on enhancing their structure for superior performance. Techniques such as site-specific conjugation and innovative radiolabeling methods have been employed to augment the binding affinity and imaging capabilities of Fab components. Importantly, the interval between injection and imaging for Fab portions has been reduced to mere hours, in contrast to days required for full antibodies, highlighting their practical advantages in clinical settings. These developments emphasize the growing relevance of fab fragments in personalized medicine, particularly in oncology, where their rapid clearance and enhanced tumor targeting can lead to more effective diagnostic and treatment strategies.
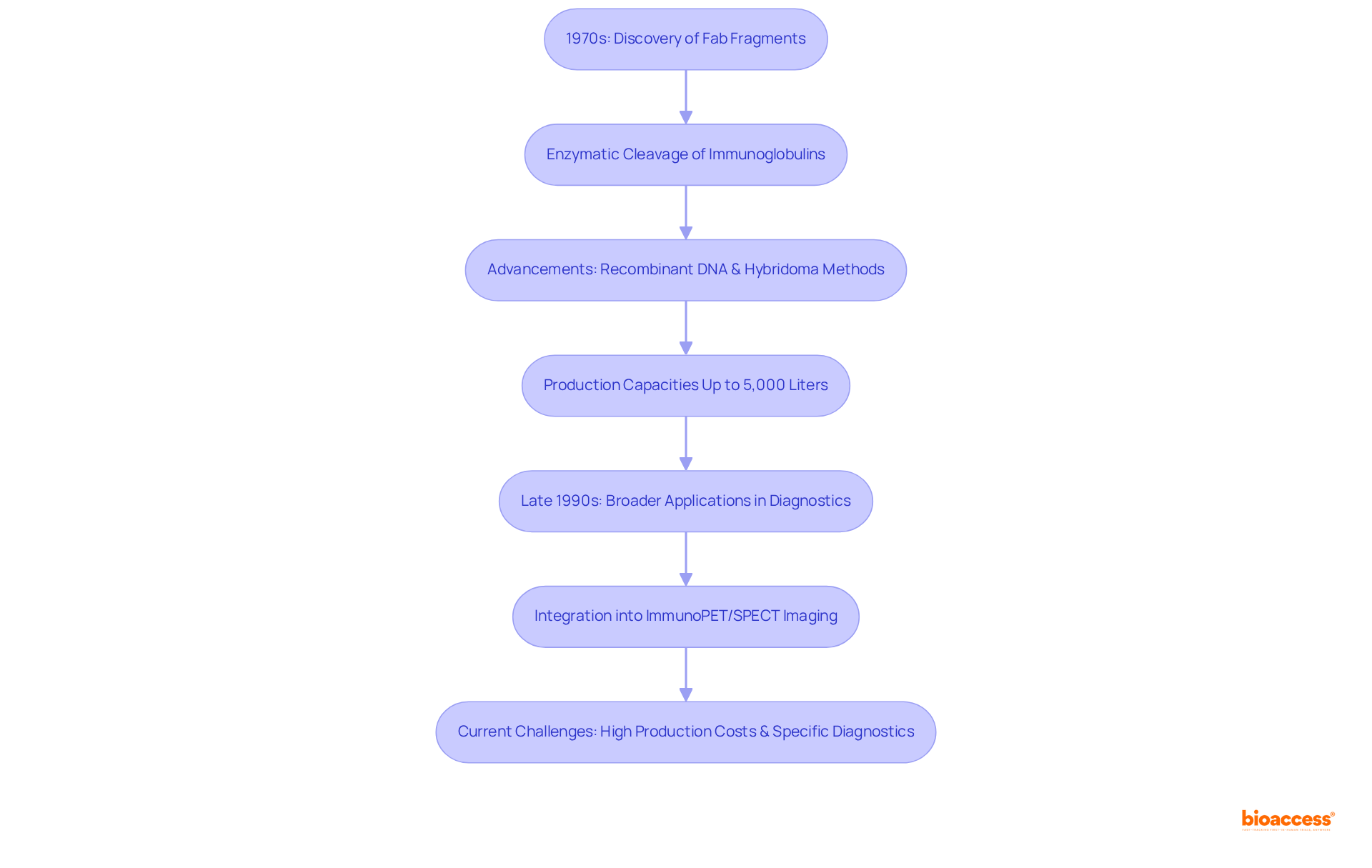
The emergence of fab fragments in the 1970s marked a significant milestone in therapeutic research. Pioneering work centered on the enzymatic cleavage of complete immunoglobulins using proteases such as papain, successfully isolating antigen-binding regions while discarding the Fc portion. This innovative method established a foundation for further exploration into fab fragment techniques.
As research progressed, recombinant DNA technology and hybridoma methods emerged, greatly enhancing the production capacities of fab fragments, which can now be manufactured at scales reaching up to 5,000 liters. By the late 1990s, the application of these components broadened, leading to their integration into diverse diagnostic and therapeutic modalities, including immunoPET/SPECT imaging techniques that utilize Fab units for targeted cancer diagnostics.
Notably, research demonstrated that enzymatic cleavage could yield fab fragment portions with high specificity and affinity, making them indispensable in targeted therapies. For instance, the advancement of [Cu]Cu-labeled immune proteins aimed at CA6 has shown promise in imaging and treating CA6-expressing tumors.
Today, fab fragment portions are recognized as crucial elements in the progression of precision medicine, particularly in oncology and infectious disease diagnostics. Nevertheless, challenges such as elevated production costs and the necessity for specific diagnostics continue to hinder the advancement of immune therapies.
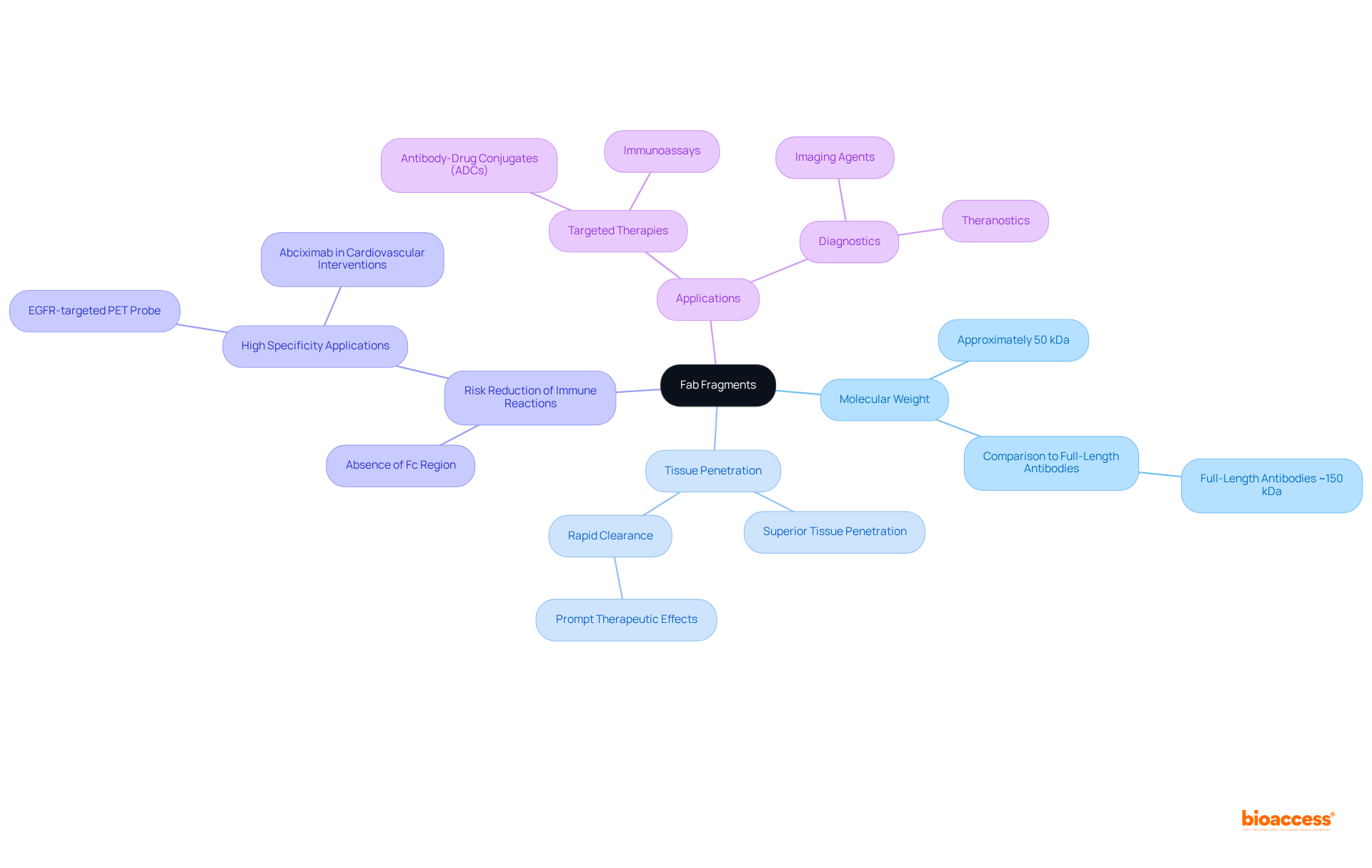
Fab fragment characteristics exhibit several key traits that enhance their medical and diagnostic applications. With a molecular weight of approximately 50 kDa, their smaller size enables superior tissue penetration and rapid clearance from the bloodstream compared to full-length antibodies, which typically weigh around 150 kDa. This swift systemic clearance is vital for achieving prompt therapeutic effects, particularly in time-sensitive treatments. The absence of the Fc region in fab fragment portions significantly reduces the risk of undesirable immune reactions and non-specific binding, making fab fragments especially suitable for applications requiring high specificity.
Moreover, fab fragments can be easily developed as components for various applications, including conjugation with drugs or imaging agents, thereby expanding their utility in targeted therapies and diagnostics. Research has demonstrated that fab fragments can effectively target specific antigens without triggering immune activation, which is particularly advantageous in managing autoimmune diseases and enhancing the precision of immunoassays. The engineering of fab fragment portions for drug delivery has yielded promising results, particularly in antibody-drug conjugates (ADCs) that combine the specificity of these components with potent cytotoxic agents, leading to improved therapeutic outcomes.
Real-world examples underscore the effectiveness of the fab fragment in clinical settings. For instance, the development of an EGFR-targeted PET probe utilizing the fab fragment of cetuximab has exhibited high specificity and remarkable target-to-background ratios in colorectal cancer models, indicating their potential as innovative screening techniques for high-risk individuals. Additionally, the application of abciximab, a fab fragment, in cardiovascular interventions has significantly improved patient outcomes by effectively preventing thrombosis during high-risk procedures. Clinical studies have shown that the fab fragment abciximab markedly reduces the incidence of thrombotic events, highlighting its clinical significance. Furthermore, Ranibizumab, a fab fragment, has been demonstrated in clinical trials to provide substantial improvements in visual clarity for individuals with retinal issues, further emphasizing the benefits of fab fragments. These examples illustrate the increasing recognition of fab fragments as vital resources in contemporary treatment and diagnostic strategies.
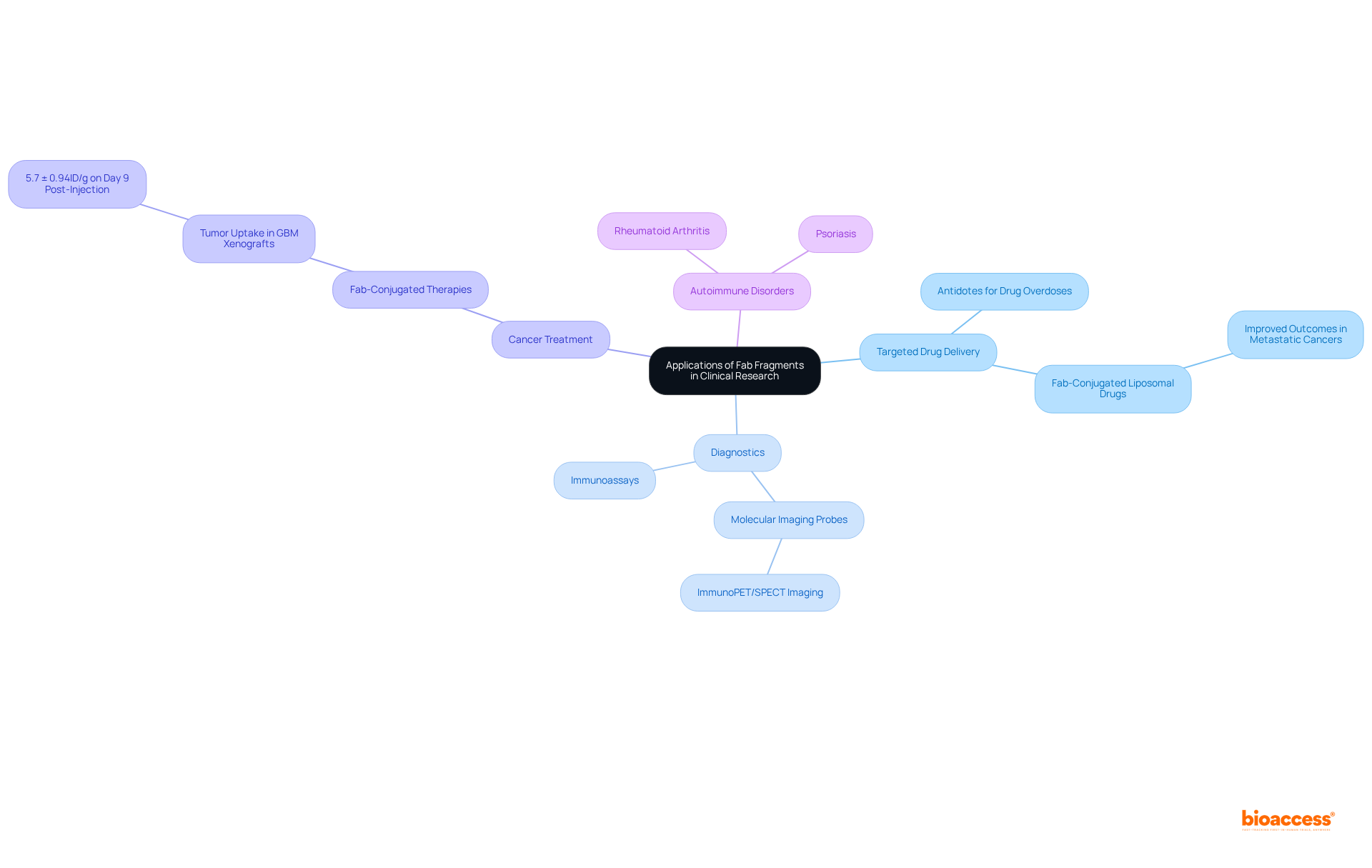
Fab components are pivotal in various clinical research applications, particularly in targeted drug delivery, diagnostics, and treatment interventions. They have been developed as antidotes for specific drug overdoses, such as digoxin toxicity, effectively neutralizing the drug's effects by binding to it in circulation. Their high specificity and low background noise render Fab portions ideal for immunoassays and diagnostic tests.
In cancer treatment, Fab units are being explored as carriers for transporting cytotoxic agents directly to tumor cells, significantly enhancing therapeutic effectiveness while minimizing systemic toxicity. Recent studies indicate that Fab-conjugated therapies have yielded improved outcomes in patients with metastatic cancers, with notable results such as a tumor uptake of 5.7 ± 0.94%ID/g for Zr-Miltuximab in GBM xenografts on day 9 post-injection.
Furthermore, Fab portions are under investigation for their roles in managing autoimmune disorders like rheumatoid arthritis and psoriasis, demonstrating their versatility beyond cancer treatment. The small size of Fab components enhances tissue penetration, facilitating effective targeting of dense tumor environments.
As research progresses, the versatility and efficacy of Fab portions continue to establish them as invaluable tools in advancing clinical research and therapeutic strategies. Notably, as of April 2025, at least eight fab fragment antibody drugs have been approved worldwide, underscoring their increasing importance in modern medicine.
Fab fragments signify a groundbreaking advancement in immunology and therapeutic applications. By isolating the antigen-binding domains of antibodies, these smaller, more efficient components provide substantial advantages in specificity and tissue penetration. The unique structure of Fab fragments not only enhances their performance in diagnostics and targeted therapies but also minimizes the risk of adverse immune responses, establishing their value in modern medicine.
In this article, we have explored key insights into the definition, origins, and characteristics of Fab fragments. From their development in the 1970s through enzymatic cleavage techniques to their current applications in precision medicine, Fab fragments have demonstrated their worth across various clinical settings. Their capacity to facilitate rapid systemic clearance and high specificity opens new avenues for targeted cancer treatments, autoimmune disorder management, and innovative diagnostic methods.
Reflecting on the significance of Fab fragments, it is evident that their role in advancing personalized medicine is paramount. As research continues to evolve, the potential for Fab fragments to transform therapeutic strategies and enhance patient outcomes is immense. Embracing these advancements in clinical research will pave the way for more effective and tailored treatment options, reinforcing the critical importance of Fab fragments in the future of healthcare.
What are Fab fragments?
Fab fragments, or antigen-binding domains, are components of immunoglobulins that retain the ability to bind to specific antigens. Each Fab portion consists of one constant and one variable domain derived from both the heavy and light chains of an antibody, connected by disulfide bonds.
How do Fab fragments differ from full antibodies?
Fab fragments exclude the Fc region of antibodies, which is responsible for mediating effector functions like complement activation and binding to Fc receptors. This absence reduces immunogenicity and enhances tissue penetration, making Fab fragments advantageous in medical applications.
What are the benefits of using Fab fragments in clinical settings?
Fab fragments have lower immunogenicity and improved tissue penetration. They have been shown to enhance pharmacokinetics and therapeutic efficacy, making them useful for targeted imaging and treatment of tumors.
Can you provide an example of a Fab fragment used in medicine?
Ranibizumab is an example of a Fab fragment approved in 2006 for the treatment of wet age-related macular degeneration, demonstrating the clinical utility of these components in improving patient outcomes.
How many Fab antibody drugs have received global approval as of April 2025?
At least eight Fab antibody drugs have received global approval, highlighting their significance in modern medicine.
What advancements have been made in the therapeutic applications of Fab fragments?
Recent advancements include enhancing their structure through techniques like site-specific conjugation and innovative radiolabeling methods, which improve binding affinity and imaging capabilities.
What is the advantage of Fab fragments in terms of imaging time?
The interval between injection and imaging for Fab portions has been reduced to mere hours, compared to the days required for full antibodies, emphasizing their practical advantages in clinical settings.
Why are Fab fragments relevant in personalized medicine, particularly in oncology?
Their rapid clearance and enhanced tumor targeting can lead to more effective diagnostic and treatment strategies in personalized medicine, making them increasingly relevant in oncology.