


The key differences in drug approval and research impact between the FDA and EMA stem from their distinct regulatory frameworks. The FDA employs a flexible, risk-based approach, enabling expedited access to new therapies. In contrast, the EMA upholds stricter requirements that ensure comprehensive safety and efficacy data prior to authorization. This distinction significantly influences market access timelines. The FDA’s expedited pathways can markedly shorten development times, while the EMA’s thorough vetting process, although potentially causing delays in approvals, enhances the credibility and acceptance of medications across Europe.
The intricate landscape of drug approval is significantly shaped by the regulatory frameworks of the FDA and EMA, two pivotal agencies governing the safety and efficacy of medications across the United States and Europe. As stakeholders in clinical research navigate these frameworks, they encounter distinct opportunities and challenges that can greatly influence the development and market entry of new therapies.
How do these differences in regulatory approaches affect not only the speed of drug approvals but also the overall credibility and acceptance of new treatments in diverse markets?
The FDA (Food and Drug Administration) and EMA (European Medicines Agency) operate under the Federal Food, Medicine, and Cosmetic Act, emphasizing the safety and effectiveness of medications and medical devices in the United States. This agency employs a risk-based strategy, allowing for adaptability in the evaluation process, which can expedite access to new therapies.
In contrast, the regulatory frameworks of the FDA and EMA standardize drug authorization across EU member states. This framework is more stringent, necessitating comprehensive data on safety and efficacy prior to authorization. Consequently, the EMA's review times are typically longer, reflecting its commitment to maintaining high patient safety standards across diverse populations.
For instance, while the FDA has projected 750 total ANDA authorizations for FY 2025, the EMA's rigorous requirements may lead to fewer but more thoroughly vetted endorsements.
Understanding the regulatory frameworks established by the FDA and EMA is crucial for stakeholders in clinical research, as they shape the medication development landscape and influence timelines and strategies for market entry.
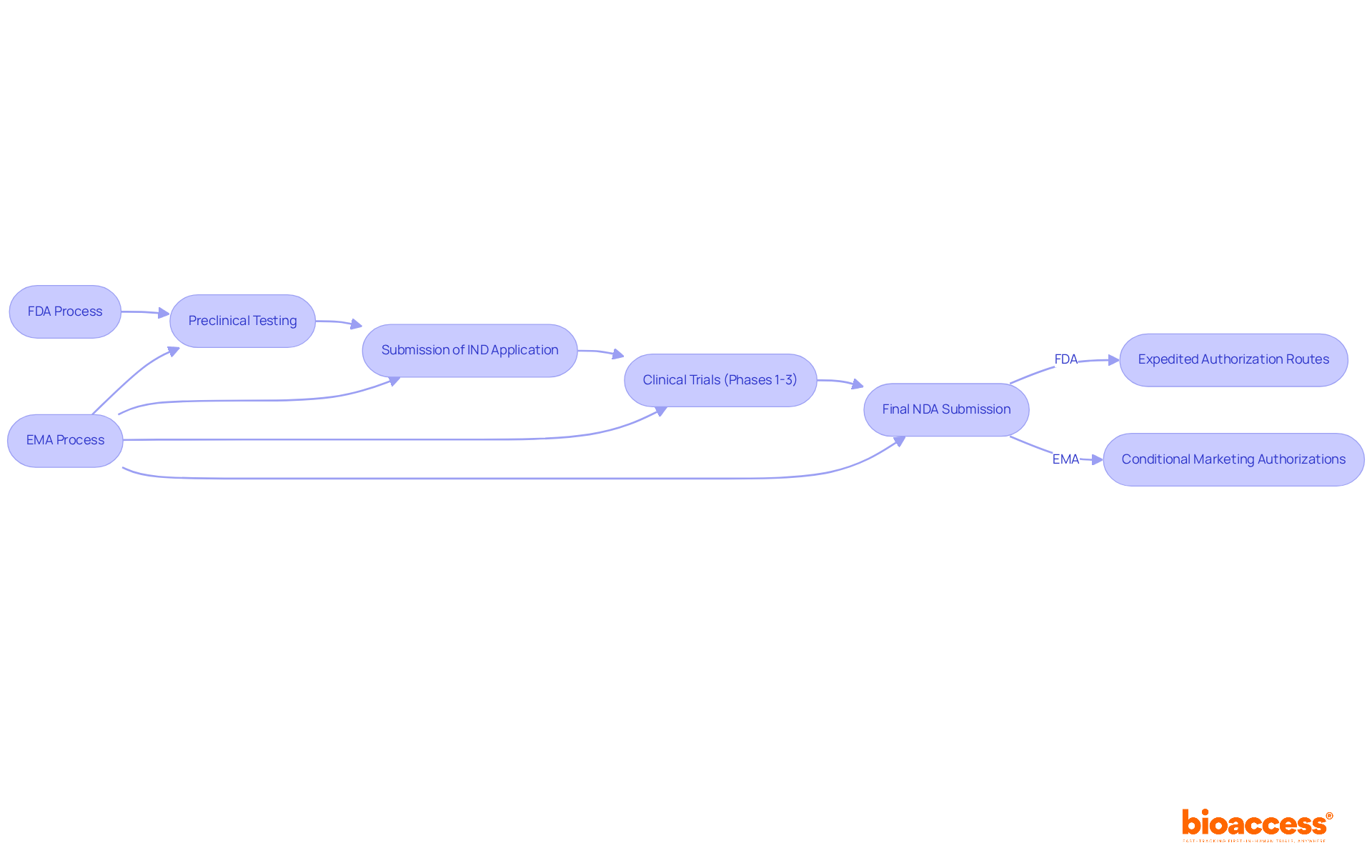
The medication authorization process by the FDA and EMA encompasses several essential stages:
Notably, the FDA facilitates expedited authorization routes, enabling faster access to medications that address unmet medical needs. In contrast, the FDA and EMA adhere to a similar sequence but emphasize a more collaborative approach with applicants throughout the development stages. The EMA offers conditional marketing authorizations, which can expedite access to promising therapies while additional data is collected.
This distinction in processes can lead to significant differences in the timelines for drugs reaching the market, thereby influencing strategic planning for pharmaceutical companies. For instance, while the FDA and EMA typically process IND applications at a pace of 1,500 to 2,000 each year, the EMA's cooperative framework may result in quicker feedback cycles, potentially shortening the overall timeline for authorization. As of 2025, the EMA's approach is increasingly relevant, especially as industry leaders advocate for more efficient pathways to deliver innovative therapies to patients.
Understanding these regulatory frameworks is crucial for companies like bioaccess®, which specializes in managing clinical trials, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH). The variations in consent procedures can directly impact the planning and execution of clinical trials, making it imperative for clinical research directors to navigate these pathways effectively.
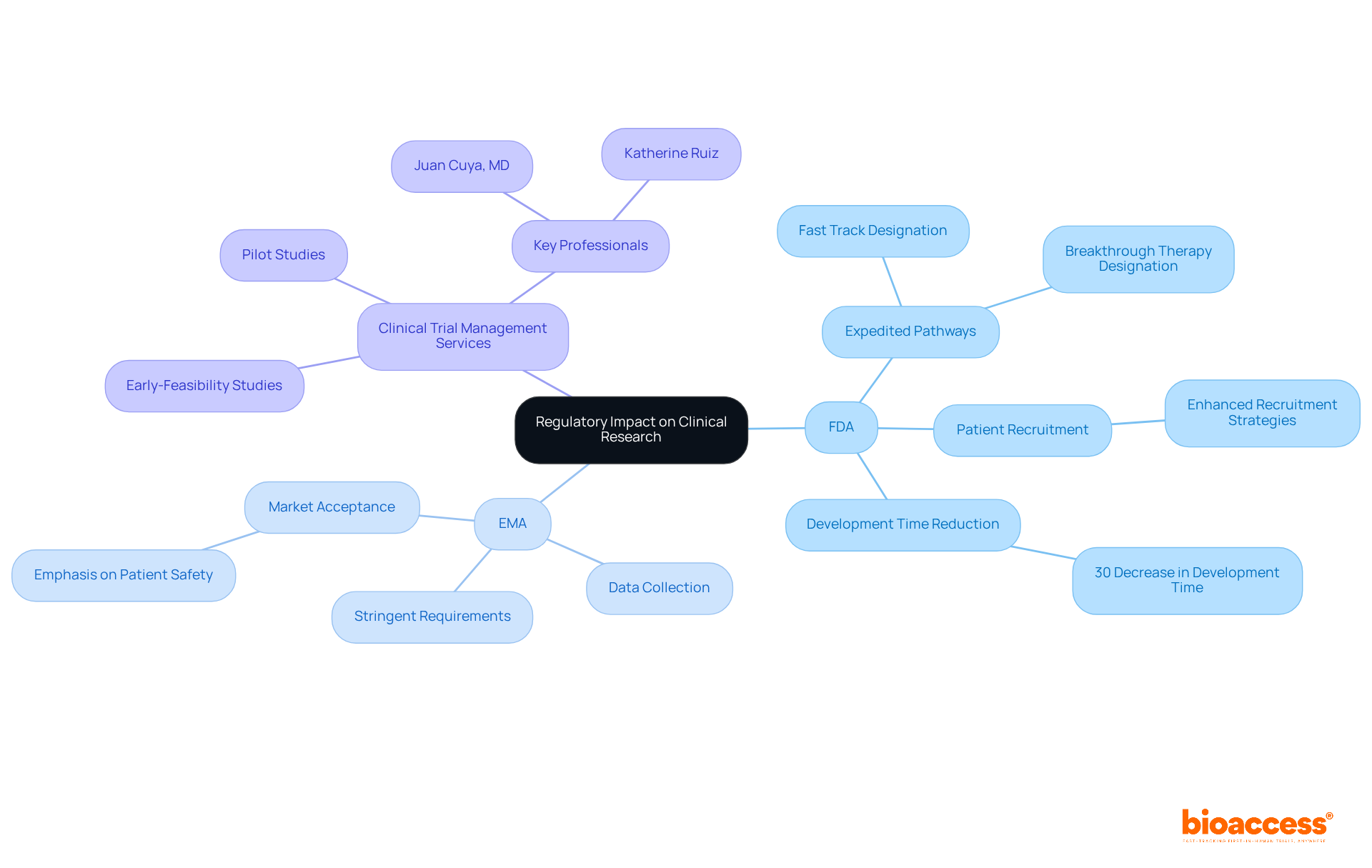
The regulatory frameworks established by the FDA and EMA are pivotal in shaping clinical research methodologies and market access strategies. The FDA's expedited pathways, such as Fast Track and Breakthrough Therapy designations, significantly enhance patient recruitment and encourage innovative trial designs, particularly in urgent therapeutic areas. For instance, fast-tracked authorizations have been associated with a 30% decrease in development time, enabling companies to deliver life-saving treatments to market more rapidly. In contrast, the EMA's stringent requirements often demand comprehensive data collection and longer trial durations, which can extend the timeline for market access. This thorough approach, while potentially delaying approvals, enhances the credibility of sanctioned medications, promoting increased market acceptance in Europe. The EMA's emphasis on patient safety and effectiveness has led to a greater proportion of medications receiving favorable assessments, resulting in improved commercial outcomes.
Understanding these regulatory effects is crucial for Medtech, Biopharma, and Radiopharma firms as they navigate the complexities of global therapeutic development and commercialization, ensuring they align their strategies with the unique demands of each market. In this context, comprehensive clinical trial management services, such as those provided by bioaccess®, play a vital role. Their expertise in conducting Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, along with services like feasibility assessments, research site selection, compliance reviews, trial setup, import permits, project management, and reporting, ensures that trials are not only compliant with regulatory standards but also strategically designed to meet the unique challenges posed by the FDA and EMA.
With professionals like Juan Cuya, MD, specializing in regulatory affairs and product launches, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, bioaccess® is well-equipped to navigate the complexities of global drug development and commercialization. Recognizing these impacts is essential for Medtech, Biopharma, and Radiopharma companies as they strive to optimize their clinical research strategies in alignment with regulatory expectations.
The regulatory landscapes of the FDA and EMA are pivotal in shaping the approval processes for new medications and medical devices. Both agencies prioritize patient safety and efficacy; however, their differing approaches to drug evaluation result in significant variations in timelines and methodologies. The FDA's risk-based strategy facilitates quicker access to therapies, while the EMA's stringent requirements ensure thorough vetting, often leading to longer review periods.
Key distinctions between the FDA and EMA processes have been highlighted throughout this article, including their respective stages of drug approval and implications for clinical research. The FDA’s expedited pathways, such as Fast Track and Breakthrough Therapy designations, contrast sharply with the EMA’s emphasis on comprehensive data collection. This ultimately influences market access strategies for pharmaceutical companies. Such differences underscore the necessity for stakeholders to adapt their clinical trial designs and regulatory strategies to align with the specific demands of each agency.
Recognizing the impact of these regulatory frameworks is essential for Medtech, Biopharma, and Radiopharma firms as they navigate the complexities of global drug development. By understanding the nuances of FDA and EMA regulations, companies can better position themselves to deliver innovative therapies effectively and efficiently. As the landscape continues to evolve, embracing these insights will be crucial in optimizing clinical research strategies and enhancing market access across diverse regions.
What are the main roles of the FDA and EMA?
The FDA (Food and Drug Administration) and EMA (European Medicines Agency) regulate medications and medical devices, ensuring their safety and effectiveness in the United States and European Union, respectively.
How does the FDA's regulatory approach differ from that of the EMA?
The FDA employs a risk-based strategy that allows for adaptability in the evaluation process, potentially expediting access to new therapies. In contrast, the EMA has a more stringent framework that requires comprehensive safety and efficacy data before authorization, leading to longer review times.
Why might the EMA have longer review times compared to the FDA?
The EMA's commitment to maintaining high patient safety standards across diverse populations necessitates comprehensive data prior to authorization, resulting in typically longer review times.
How many ANDA authorizations does the FDA project for FY 2025?
The FDA has projected 750 total ANDA (Abbreviated New Drug Application) authorizations for FY 2025.
What is the significance of understanding the regulatory frameworks of the FDA and EMA for stakeholders in clinical research?
Understanding these frameworks is crucial for stakeholders as they shape the medication development landscape and influence timelines and strategies for market entry.