


This article outlines the critical steps necessary for achieving compliance with medical device labeling regulations in Mexico, particularly under the updated NOM-137-SSA1-2024. It underscores the necessity of comprehensive documentation and strict adherence to COFEPRIS guidelines. Furthermore, it highlights the proactive management of common labeling challenges as essential for ensuring successful market entry and safeguarding patient safety.
Navigating the intricate landscape of medical device labeling in Mexico is essential for manufacturers aiming to enter this burgeoning market. With the updated NOM-137-SSA1-2024 regulations set to take effect in July 2025, understanding the compliance requirements is more crucial than ever. This guide offers valuable insights into the steps necessary for successful labeling, from gathering required documentation to troubleshooting common issues that may arise.
However, with stringent regulations and potential pitfalls at every turn, manufacturers must consider:
To effectively label medical products in Mexico, a free guide device labeling Mexico to understand the regulatory landscape established by COFEPRIS (Federal Commission for Protection against Sanitary Risks) is paramount. The updated NOM-137-SSA1-2024, effective from July 2025, provides a free guide device labeling Mexico that outlines crucial marking requirements, including:
Compliance with these regulations, particularly the free guide device labeling Mexico, is not merely mandatory; it is vital for avoiding legal complications and ensuring timely market entry. The standard mandates that all medical equipment must include:
Furthermore, medical device kits must list registration numbers for all components and adhere to labeling requirements, ensuring that all information is accessible and comprehensible. As noted by COFEPRIS, "Failure to comply with these regulations can lead to significant delays in product approval and market entry." By aligning with these standards, manufacturers can facilitate smoother regulatory procedures and enhance patient safety.
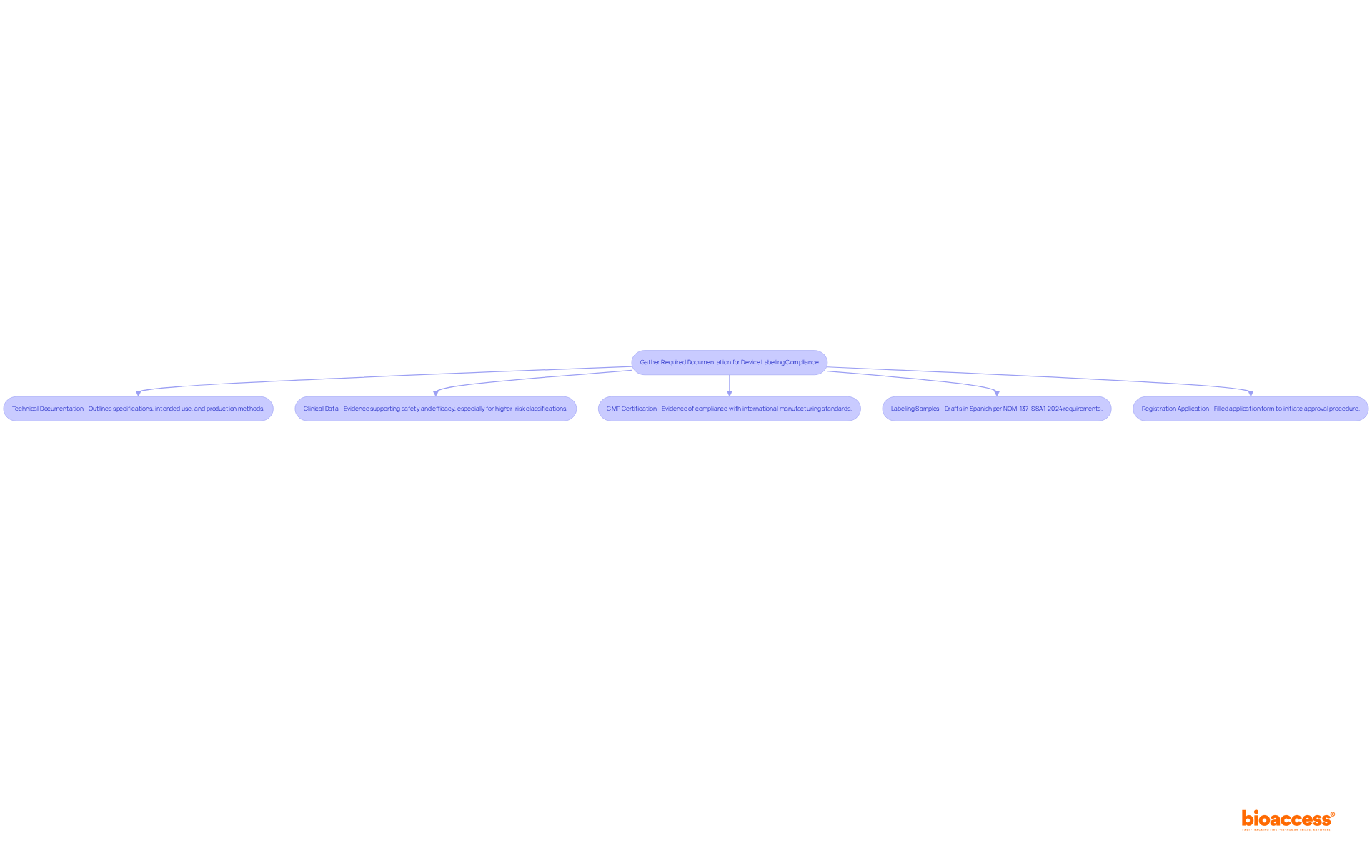
To comply with Mexico's labeling regulations, a free guide device labeling Mexico for compiling several key documents is essential.
Technical Documentation is paramount; it must thoroughly outline the specifications, intended use, and production methods. Regulatory specialists emphasize that proper technical documentation is crucial for ensuring compliance of equipment and facilitating smoother approval processes. In fact, 32% of FDA 510(k) submissions failed the minimum acceptability check in 2022, underscoring the importance of thorough documentation.
Clinical Data is another critical component, including evidence that supports the safety and efficacy of the instrument, particularly for higher-risk classifications (Class II, III, and IV), which generally have more stringent requirements for clinical data. Medical devices in Mexico are classified into four classes (I, II, III, and IV), and understanding these classifications is vital for compliance.
Good Manufacturing Practices (GMP) Certification is also necessary, providing evidence that the manufacturing method complies with international standards, which is essential for regulatory approval.
Labeling Samples must include drafts of the proposed labels in Spanish, incorporating all required information as per NOM-137-SSA1-2024 and the free guide device labeling Mexico, which aligns with international labeling regulations and introduces new definitions and requirements.
Lastly, a Registration Application is necessary; a filled-out application form must be submitted to the health authority to initiate the approval procedure.
Ensuring that all documents are precise and current is essential, as this can significantly decrease the chances of delays in the approval process. In 2025, the regulatory agency is anticipated to authorize a greater proportion of medical equipment submissions, highlighting the significance of detailed and compliant technical documentation.
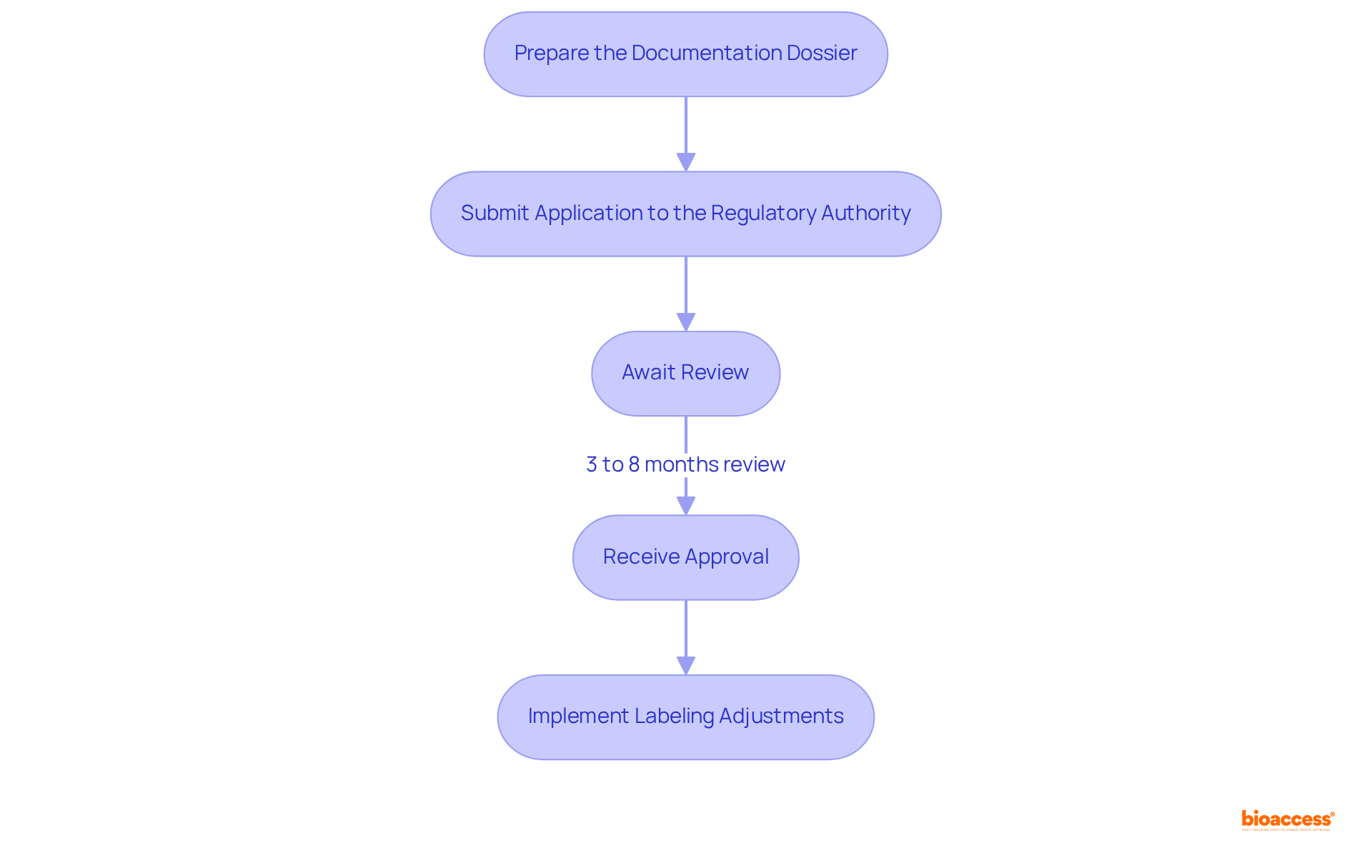
The device labeling process in Mexico is a critical pathway to ensure compliance with COFEPRIS regulations, comprising several essential steps:
Understanding the success rates of medical labeling applications in Mexico, including the free guide device labeling Mexico, is essential, particularly as the market is anticipated to reach US$10.18 billion by 2030, with a compound annual growth rate of 6.31% from 2025. Industry leaders emphasize the importance of thorough preparation and adherence to regulatory requirements to enhance the likelihood of successful submissions. As pointed out by Ana Criado, "It is crucial for manufacturers to remain aware of the medical equipment regulations in Mexico and any alterations in this regulatory environment to sustain their market position efficiently." Following these steps diligently will not only streamline the approval process but also position your product favorably in the growing Mexican medical devices market.
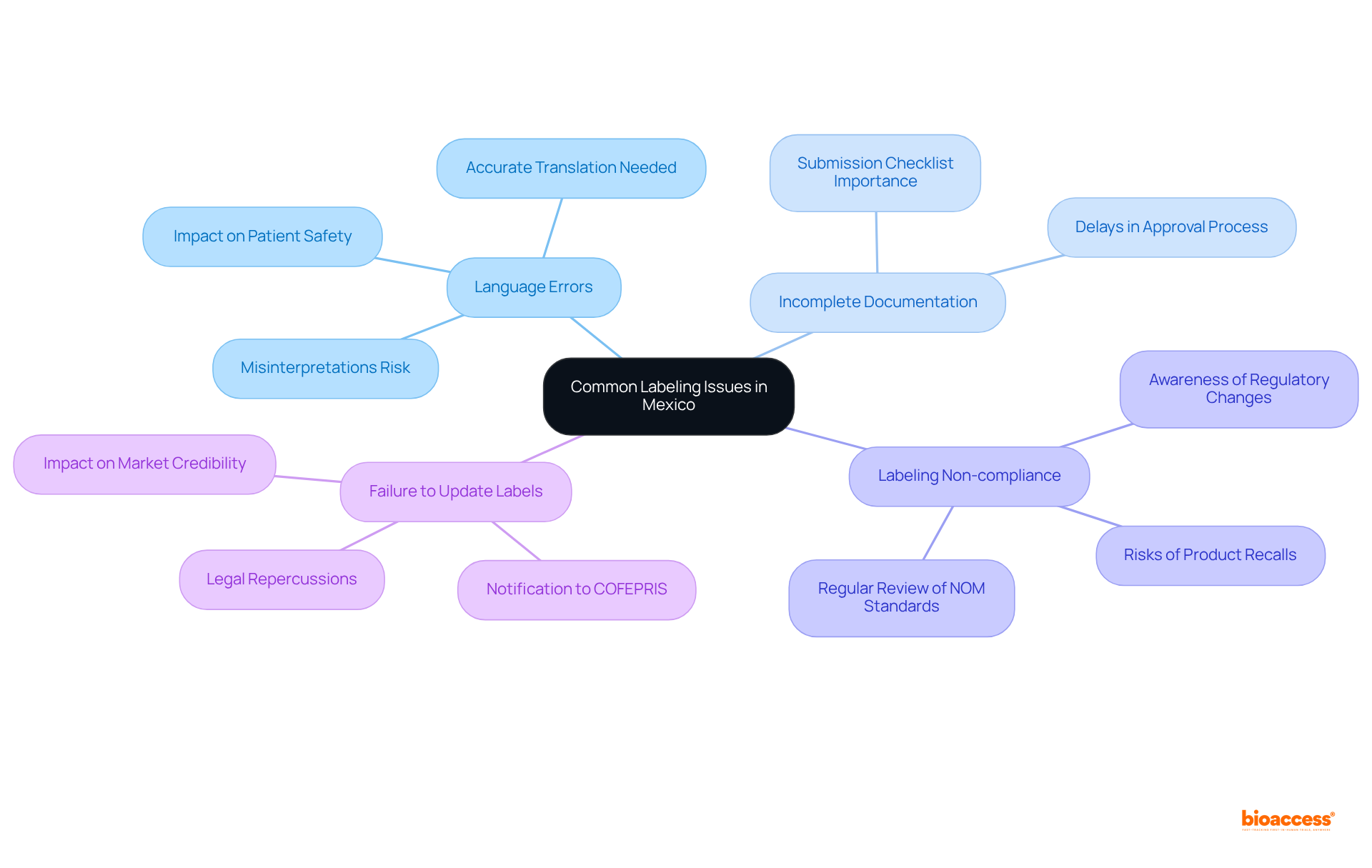
Common labeling issues in Mexico include:
Language Errors: Accurate translation of labels and instructions for use (IFU) into Spanish is crucial. Misinterpretations can lead to significant compliance failures, as evidenced by studies showing that only 3% of university nutrition students can correctly interpret existing labels. This underscores the necessity for clear communication to avert misunderstandings that could jeopardize patient safety.
Incomplete Documentation: Ensure that all necessary documents are included in your submission to the relevant authority. Missing documents can lead to delays in the approval process, which is already time-consuming due to regulatory complexities. A thorough checklist can effectively mitigate this risk.
Labeling Non-compliance: Regularly review the latest NOM standards, particularly NOM-137-SSA1-2008, to ensure that your labels meet current requirements. The regulatory agency published a draft revision of the standard, NOM-137-SSA1-2024, in 2024, highlighting the significance of remaining informed about regulatory changes. Non-compliance can result in product recalls or fines, emphasizing the need for vigilance in this area.
Failure to Update Labels: If there are changes to the device or its intended use, it is essential to update the labels accordingly and notify COFEPRIS. Neglecting to do so can lead to legal repercussions, including fines and operational shutdowns, undermining the credibility of your product in the market.
By proactively addressing these issues, you can simplify the tagging process and enhance your chances of successful compliance with the free guide device labeling Mexico in the evolving regulatory landscape. Moreover, with approximately 10% of Mexicans having some form of diabetes, the importance of free guide device labeling Mexico for health-related products cannot be overstated. The growing medical device market in Mexico further underscores the urgency of addressing these compliance issues.
Understanding and complying with Mexico's medical device labeling regulations is crucial for manufacturers aiming to enter this burgeoning market. This article emphasizes the importance of adhering to the updated NOM-137-SSA1-2024 standards set by COFEPRIS, which outline specific labeling requirements that ensure not only legal compliance but also the safety and efficacy of medical products. By following these guidelines, manufacturers can navigate the complexities of the regulatory landscape and facilitate a smoother product approval process.
Key points discussed include:
By being proactive in these areas, companies can significantly enhance their chances of successful market entry and avoid costly delays.
As the Mexican medical device market continues to expand, the significance of understanding and implementing proper labeling practices cannot be overstated. Manufacturers are encouraged to utilize available resources, such as the free guide on device labeling in Mexico, to stay informed and compliant with evolving regulations. Embracing these guidelines not only ensures legal adherence but also promotes patient safety and trust in medical products, ultimately contributing to the overall growth and integrity of the healthcare sector in Mexico.
What is the importance of understanding Mexico's regulatory framework for medical device labeling?
Understanding Mexico's regulatory framework is crucial for effectively labeling medical products and ensuring compliance with COFEPRIS regulations, which helps avoid legal complications and facilitates timely market entry.
What is the updated regulation for medical device labeling in Mexico?
The updated regulation is NOM-137-SSA1-2024, which will be effective from July 2025.
What are the key marking requirements outlined in the free guide for device labeling in Mexico?
The key marking requirements include the item's name, intended use, safety instructions, and any necessary health information.
What additional information must be included on medical equipment labels?
Labels must include clear identification of the manufacturer, technical descriptions, and any residual risks associated with the use of the equipment.
What specific requirements apply to medical device kits in terms of labeling?
Medical device kits must list registration numbers for all components and adhere to labeling requirements to ensure that all information is accessible and comprehensible.
What are the consequences of failing to comply with these labeling regulations?
Failure to comply can lead to significant delays in product approval and market entry, as noted by COFEPRIS.