Overview
To obtain a quote for an Authorized Representative (AR) for medical devices in Mexico, manufacturers must engage a legally registered AR to effectively navigate regulatory compliance with COFEPRIS. This article highlights the essential roles of the AR, which include:
- Managing documentation
- Serving as a regulatory liaison
- Ensuring adherence to safety standards
These responsibilities are critical for facilitating a smooth registration process and securing market access.
Introduction
Navigating the intricate landscape of medical device regulations in Mexico is crucial for foreign manufacturers seeking to penetrate this lucrative market. The role of an Authorized Representative (AR) is essential, serving as the vital link between manufacturers and COFEPRIS, the regulatory authority. This article explores the pivotal steps required for achieving compliance, emphasizing the advantages of selecting the appropriate AR and preparing the necessary documentation. With regulations continually evolving and the risk of costly delays, manufacturers must consider:
- How can they ensure a seamless and efficient authorization process?
Understand the Role of an Authorized Representative in Mexico
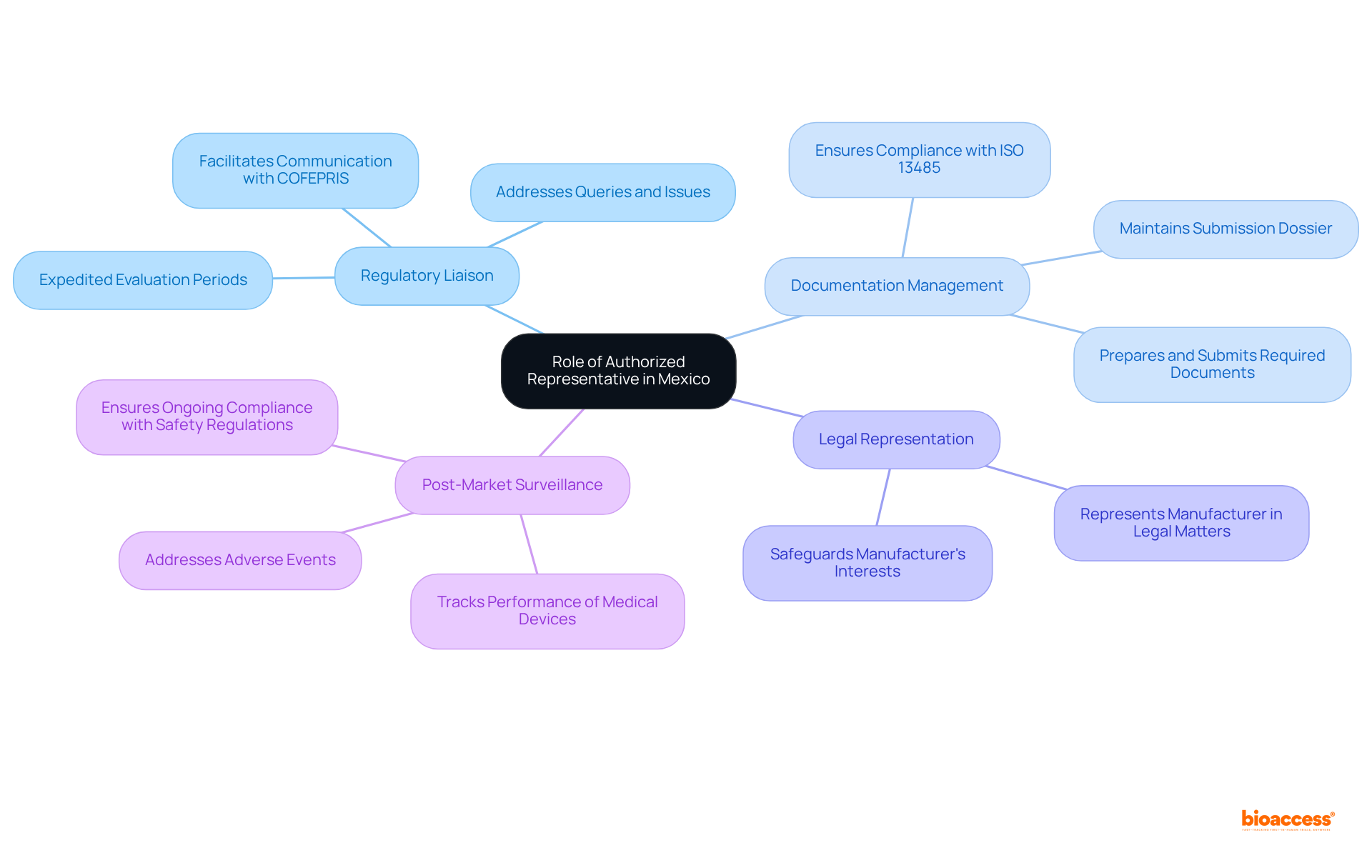
In Mexico, to get quote authorized representative mexico devices, an Authorized Representative (AR) serves as a legally registered entity responsible for ensuring the regulatory adherence of medical devices for foreign manufacturers. Acting as the primary liaison with COFEPRIS (the Federal Commission for Protection against Sanitary Risks), the AR plays a pivotal role in submitting all necessary documentation accurately and effectively representing the manufacturer's interests in regulatory matters to get quote authorized representative mexico devices. The key responsibilities of an AR include:
- Regulatory Liaison: The AR facilitates communication with COFEPRIS throughout the registration process, addressing any queries or issues that may arise. This role is crucial, especially considering that the evaluation period for health equipment registration is anticipated to range from 3 to 8 months in 2025. Notably, this period may be expedited to just 1 to 3 months through the engagement of an Authorized Third Party.
- Documentation Management: They meticulously prepare, submit, and maintain all required documents in accordance with local regulations. This includes ensuring that the submission dossier comprises technical specifications, clinical data, and proof of adherence to ISO 13485 standards.
- Legal Representation: The AR represents the manufacturer in legal matters related to product registration and adherence, safeguarding their interests while navigating the complexities of the regulatory landscape.
- Post-Market Surveillance: Additionally, the AR is accountable for tracking the performance of medical instruments after they have received approval, ensuring ongoing adherence to safety regulations and addressing any adverse events.
The significance of ARs cannot be overstated, as they play a crucial role in enhancing public health outcomes and fostering innovation within the medical equipment sector. Their diverse duties guarantee adherence to regulatory standards while enabling market access for medical devices. Successful examples of regulatory adherence managed by authorized representatives in Mexico demonstrate their effectiveness in navigating the approval procedure for devices, which involves a structured six-step evaluation requiring a comprehensive submission dossier, allowing companies to get quote authorized representative Mexico devices. By comprehending the critical functions of an AR, manufacturers can ensure a smoother registration process and mitigate potential compliance pitfalls. As Ana Criado, Director of Regulatory Affairs, emphasizes, "It is essential for manufacturers to remain updated on the medical product regulations in Mexico and any alterations in this regulatory environment to sustain their market position efficiently.
Identify Regulatory Requirements for Medical Device Authorization
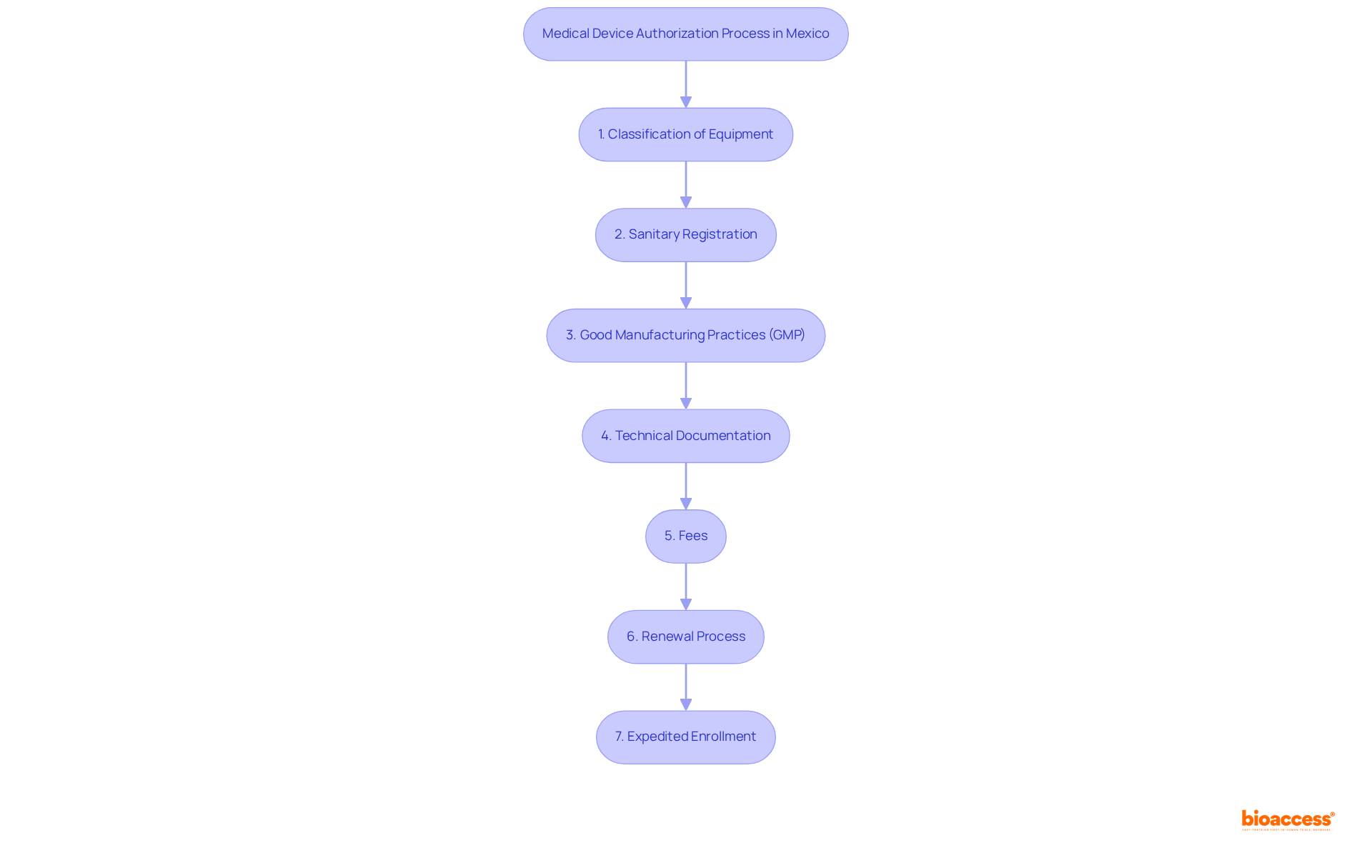
To successfully authorize a medical instrument in Mexico, manufacturers must get quote authorized representative Mexico devices and comply with several regulatory requirements established by COFEPRIS, the governing body for medical instrument regulation. Understanding these requirements is essential for a successful application to get quote authorized representative Mexico devices, as it helps in avoiding delays in the approval procedure. Key steps include:
- Classification of the Equipment: Identify the classification of your medical equipment as Class I, II, or III, based on its risk level. This classification helps to get quote authorized representative Mexico devices and outlines the regulatory pathway and specific requirements for approval.
- Sanitary Registration: Secure a Sanitary Registration Number, which is essential for all medical devices marketed in Mexico. This process involves getting a quote from an authorized representative in Mexico for the necessary devices along with submitting an application and the requisite documentation.
- Good Manufacturing Practices (GMP): Ensure adherence to GMP standards, which may necessitate certification from an accredited organization, reflecting the commitment to quality and safety. Manufacturers must adhere to ISO 13485 or equivalent standards for their Quality Management System.
- Technical Documentation: Compile a detailed technical dossier that includes clinical data, labeling information, and evidence of safety and efficacy. This documentation is critical for demonstrating compliance with regulatory standards to get quote authorized representative Mexico devices.
- Fees: Be mindful of the enrollment fees, which differ based on the device class and the complexity of the submission.
- Renewal Process: Medical equipment listings in Mexico remain valid for five years and must be renewed before expiration. The renewal procedure can take 5-8 months.
- Expedited Enrollment: Utilizing an Authorized Third Party can accelerate enrollment to as little as 1 to 3 months.
With the healthcare equipment regulatory affairs market in Mexico projected to reach USD 120.7 million by 2030, navigating these regulations effectively is more important than ever.
Select and Appoint Your Authorized Representative
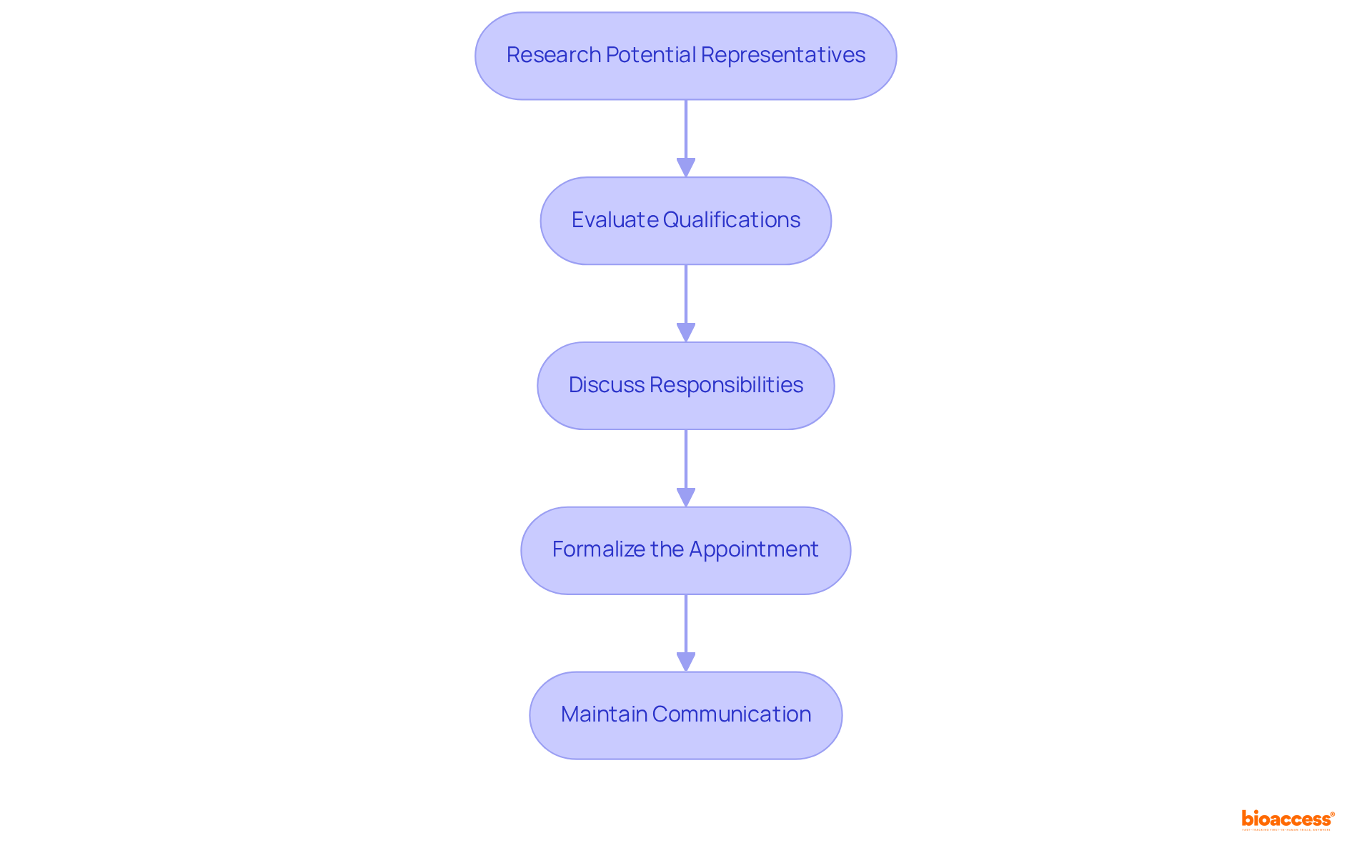
Selecting the appropriate Authorized Representative (AR) is crucial to get quote authorized representative Mexico devices and to ensure adherence in the medical product approval process in Mexico. To effectively select and appoint your AR, follow these steps:
- Research Potential Representatives: Identify ARs with a strong track record in medical equipment registration within Mexico. Assess their experience, reputation, and client testimonials to gauge their effectiveness.
- Evaluate Qualifications: Confirm that the AR possesses a comprehensive understanding of COFEPRIS regulations and the medical equipment landscape. They should be well-versed in the specific requirements pertinent to your device class.
- Discuss Responsibilities: Clearly define the responsibilities and expectations of the AR, including communication protocols, documentation management, and regulatory representation. This clarity will help avoid misunderstandings later on.
- Formalize the Appointment: After selecting an AR, formalize the relationship through a written agreement that outlines the terms of engagement, including fees and the duration of service. This contract should protect both parties and ensure accountability.
- Maintain Communication: Establish a routine for regular communication with your AR to promptly address any regulatory updates and requirements. This continuous conversation is essential for managing the intricacies of the enrollment procedure.
To successfully enter Mexico's expanding medical device industry and potentially attain USD 9.72 billion by 2028, it is crucial to get quote authorized representative Mexico devices, as choosing an informed and dependable AR not only simplifies the enrollment procedure but also greatly improves adherence. Furthermore, collaborating with seasoned ARs may result in quicker regulatory approvals, as bioaccess® secures ethical approvals in merely 4-6 weeks, significantly enhancing the efficiency of the submission.
Prepare Required Documentation and Ensure Compliance
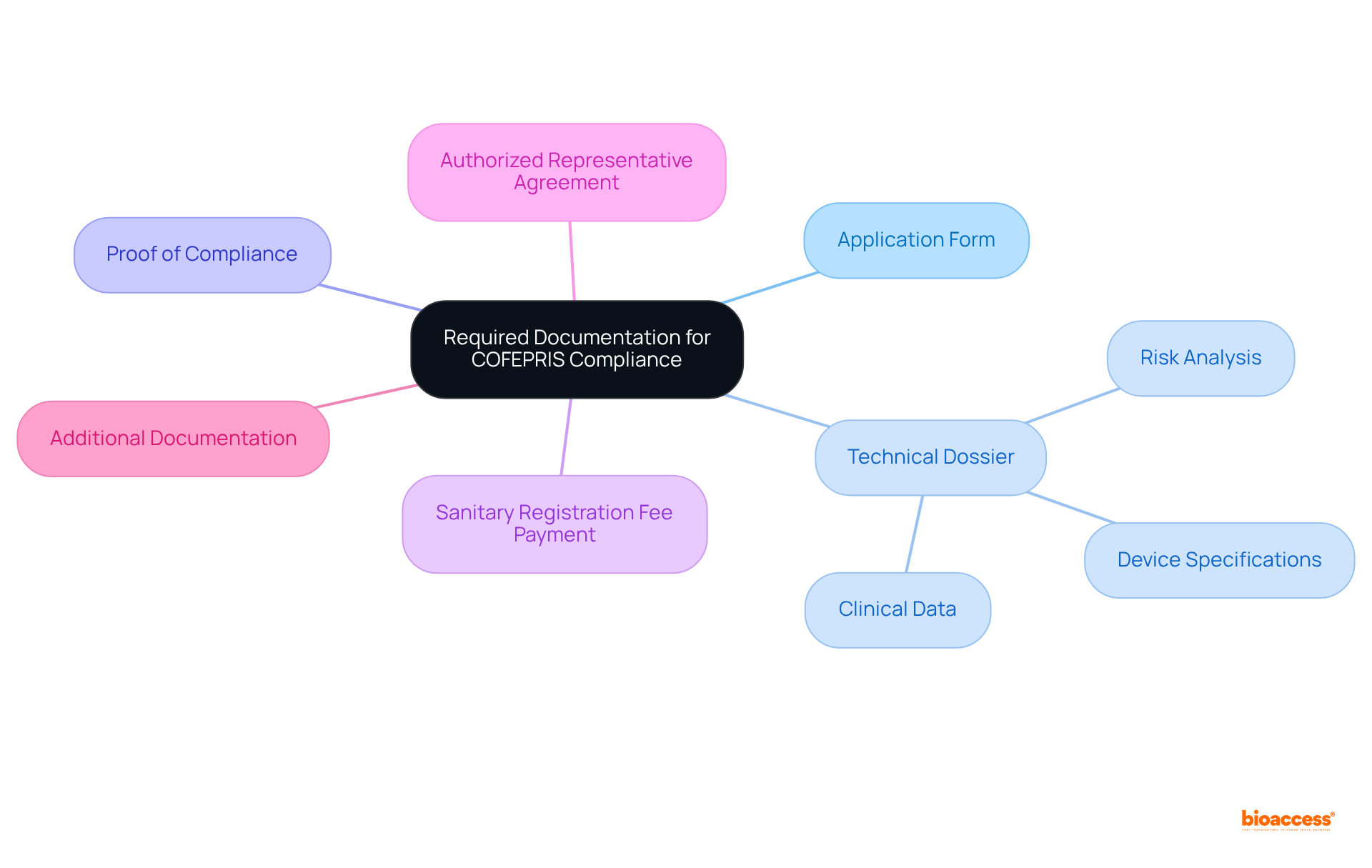
To ensure compliance and facilitate a successful enrollment process with COFEPRIS, meticulous preparation of the required documentation is crucial. Below is a comprehensive checklist of the necessary documents:
- Application Form: Complete the COFEPRIS application form for medical product registration.
- Technical Dossier: Include detailed technical documentation demonstrating the safety and efficacy of the device, encompassing:
- Clinical data (if applicable)
- Risk analysis and management documentation
- Device specifications and labeling information
- Proof of Compliance: Provide evidence of compliance with Good Manufacturing Practices (GMP) or equivalent standards, such as ISO 13485. To proceed, please get quote authorized representative Mexico devices certification. While ISO 13485 is not technically necessary for certification, evidence of an audited quality system is essential.
- Sanitary Registration Fee Payment: Include proof of payment for the registration fees, which differ according to the equipment class.
- Authorized Representative Agreement: Submit a copy of the agreement with your appointed Authorized Representative, who will get quote authorized representative Mexico devices and act as your in-country representative.
- Additional Documentation: Depending on the category of the equipment, further documents may be necessary, such as a Free Sale Certificate or Foreign Government Certificate. Class I Low Risk devices typically experience faster review and approval times compared to higher classes.
Once all documentation is prepared, conduct a thorough review to ensure accuracy and completeness before submission to COFEPRIS. This diligence is essential, as it can greatly decrease the average duration required for enrollment and improve the chance of a successful result. Adhering to best practices in documentation preparation not only simplifies the workflow but also aligns with COFEPRIS compliance requirements, ultimately enabling faster access to the market. As noted by Emergo by UL, "Applications going through the standard review process are also eligible for Third Party Review, at a cost," which can further expedite the registration process.
Conclusion
The journey to compliance for medical devices in Mexico fundamentally relies on the pivotal role of an Authorized Representative (AR). This entity adeptly navigates the complexities of regulatory requirements, facilitating a more seamless pathway for foreign manufacturers to penetrate the Mexican market. Grasping the AR's responsibilities—from documentation management to post-market surveillance—is crucial for ensuring adherence to the stringent standards mandated by COFEPRIS.
The key steps delineated in the article include:
- Selection of a qualified AR
- Preparation of necessary documentation
- Understanding of the regulatory landscape
These steps underscore the necessity of diligence throughout the authorization process. Manufacturers must:
- Classify their devices
- Secure sanitary registrations
- Uphold compliance with Good Manufacturing Practices
to achieve successful market entry. The emphasis on meticulous documentation preparation is paramount, as it directly influences the efficiency of the approval process.
As the medical device market in Mexico continues to expand, the importance of engaging a knowledgeable and reliable Authorized Representative becomes increasingly evident. Manufacturers are urged to prioritize compliance and remain vigilant regarding regulatory changes to sustain their competitive advantage. By doing so, they not only enhance their prospects for successful market access but also contribute positively to the overall improvement of public health outcomes in Mexico.
Frequently Asked Questions
What is the role of an Authorized Representative (AR) in Mexico?
An Authorized Representative (AR) in Mexico is a legally registered entity responsible for ensuring regulatory adherence of medical devices for foreign manufacturers. They serve as the primary liaison with COFEPRIS and represent the manufacturer's interests in regulatory matters.
What are the key responsibilities of an Authorized Representative?
The key responsibilities of an AR include facilitating communication with COFEPRIS, managing documentation, providing legal representation, and conducting post-market surveillance of medical devices.
How does an AR facilitate communication with COFEPRIS?
The AR addresses queries or issues that may arise during the registration process with COFEPRIS, ensuring effective communication throughout the evaluation period.
What is the expected evaluation period for health equipment registration in Mexico?
The evaluation period for health equipment registration is anticipated to range from 3 to 8 months in 2025, but it may be expedited to just 1 to 3 months with the engagement of an Authorized Third Party.
What types of documents does an AR manage for medical device registration?
An AR prepares, submits, and maintains required documents, including technical specifications, clinical data, and proof of adherence to ISO 13485 standards.
How does an AR provide legal representation for manufacturers?
The AR represents the manufacturer in legal matters related to product registration and adherence, helping navigate the complexities of the regulatory landscape.
What is the role of an AR in post-market surveillance?
The AR is responsible for tracking the performance of medical instruments after approval, ensuring ongoing adherence to safety regulations and addressing any adverse events.
Why are Authorized Representatives significant in the medical equipment sector?
ARs enhance public health outcomes and foster innovation by ensuring regulatory standards are met, thereby enabling market access for medical devices.
What can manufacturers do to ensure a smoother registration process in Mexico?
Manufacturers can understand the critical functions of an AR to mitigate potential compliance pitfalls and facilitate a smoother registration process.
Why is it important for manufacturers to stay updated on medical product regulations in Mexico?
Staying updated on medical product regulations and any changes in the regulatory environment is essential for manufacturers to sustain their market position efficiently.
List of Sources
- Understand the Role of an Authorized Representative in Mexico
- Mastering Medical Device Regulations Mexico: A Comprehensive Guide (https://bioaccessla.com/br/blog/mastering-medical-device-regulations-mexico-a-comprehensive-guide)
- Medical Devices - Mexico | Statista Market Forecast (https://statista.com/outlook/hmo/medical-technology/medical-devices/mexico)
- Understanding the Key Responsibilities of an Authorized Representative in the Medical Device Industry - OMC Medical (https://omcmedical.com/understanding-the-key-responsibilities-of-an-authorized-representative-in-the-medical-device-industry)
- Mexico - K. Healthcare Products & Services | Privacy Shield (https://privacyshield.gov/ps/article?id=Mexico-Healthcare-Products-Services)
- Identify Regulatory Requirements for Medical Device Authorization
- Mastering Medical Device Regulations Mexico: A Comprehensive Guide (https://bioaccessla.com/blog/mastering-medical-device-regulations-mexico-a-comprehensive-guide)
- Medical device production value Mexico 2016-2020| Statista (https://statista.com/statistics/632676/medical-device-production-in-mexico)
- Global Medical Device Podcast powered by Greenlight Guru (https://podcasts.apple.com/us/podcast/global-medical-device-podcast-powered-by-greenlight-guru/id1036394532)
- Mexico – Overview of Medical Device Industry and Healthcare Statistics (https://emergobyul.com/resources/mexico-overview-medical-device-industry-and-healthcare-statistics)
- Medical Device Manufacturing in Mexico (https://tetakawi.com/industries/medical-device)
- Select and Appoint Your Authorized Representative
- Medical Device Registrations in Mexico. Decode the Regulatory Essentials. | Freyr - Global Regulatory Solutions and Services Company (https://freyrsolutions.com/blog/medical-device-registrations-in-mexico-decode-the-regulatory-essentials)
- Ultimate Guide for Regulatory Affairs in Mexico (https://veraqueconsulting.com/mx/ultimate-guide-for-regulatory-affairs-in-mexico)
- Mexico Medical Device Registration | OMC Medical Limited (https://omcmedical.com/mexico-medical-device-registration)
- Medical device consumption value Mexico 2016-2020| Statista (https://statista.com/statistics/632690/medical-device-consumption-in-mexico)
- 4 Steps to Leverage Market Entry Accelerator Mexico Medical Devices (https://bioaccessla.com/blog/4-steps-to-leverage-market-entry-accelerator-mexico-medical-devices)
- Prepare Required Documentation and Ensure Compliance
- Medical Device Registration at Cofepris: Everything You Need to Know in 2025 (https://globalregulatorypartners.com/medical-device-registration-at-cofepris-everything-you-need-to-know-in-2025)
- COFEPRIS medical device and IVD registration and approval in Mexico (https://emergobyul.com/services/cofepris-medical-device-and-ivd-registration-and-approval-mexico)
- Mexico Medical Device and IVD Regulatory Approval Process (https://emergobyul.com/resources/mexican-regulatory-approval-process-medical-and-ivd-devices)
- Mexico Medical Device Registration | OMC Medical Limited (https://omcmedical.com/mexico-medical-device-registration)






