


To become a Clinical Research Associate (CRA), individuals must prioritize acquiring a relevant educational background, securing certifications, and gaining practical experience through internships or entry-level positions. This article outlines these essential steps, underscoring the significance of education and certifications—most notably, the Certified Clinical Research Associate (CCRA). Furthermore, it highlights the necessity of hands-on experience and tailored application materials to enhance job prospects in this burgeoning field, projected to exceed $80 billion by 2025. As the demand for skilled professionals in clinical research continues to grow, following these guidelines will position aspiring CRAs for success.
The role of a Clinical Research Associate (CRA) has become increasingly vital in the fast-evolving landscape of medical research, serving as the crucial link between sponsors and research sites. Aspiring CRAs have the opportunity to gain a wealth of knowledge regarding the responsibilities, educational pathways, and practical experiences essential for thriving in this competitive field.
But what does it truly take to navigate the complexities of becoming a CRA?
How can one effectively position themselves for success amidst the growing demand for these professionals?
Exploring these essential steps not only illuminates the career path but also reveals the opportunities that await those ready to embark on this journey.
A Clinical Research Associate (CRA) plays a pivotal role in the research process, serving as a vital link between sponsors and research sites. Their key responsibilities encompass several critical areas:
In 2025, the demand for Clinical Research Associates is projected to increase significantly, with the clinical trial market expected to surpass 70 billion dollars. Understanding these responsibilities and the associated challenges is crucial for anyone learning how to become a clinical research associate, as it aligns with the skills and interests necessary for success in this dynamic field.

To thrive as a Clinical Research Associate (CRA), knowing how to become a clinical research associate requires a solid educational foundation and relevant certifications.
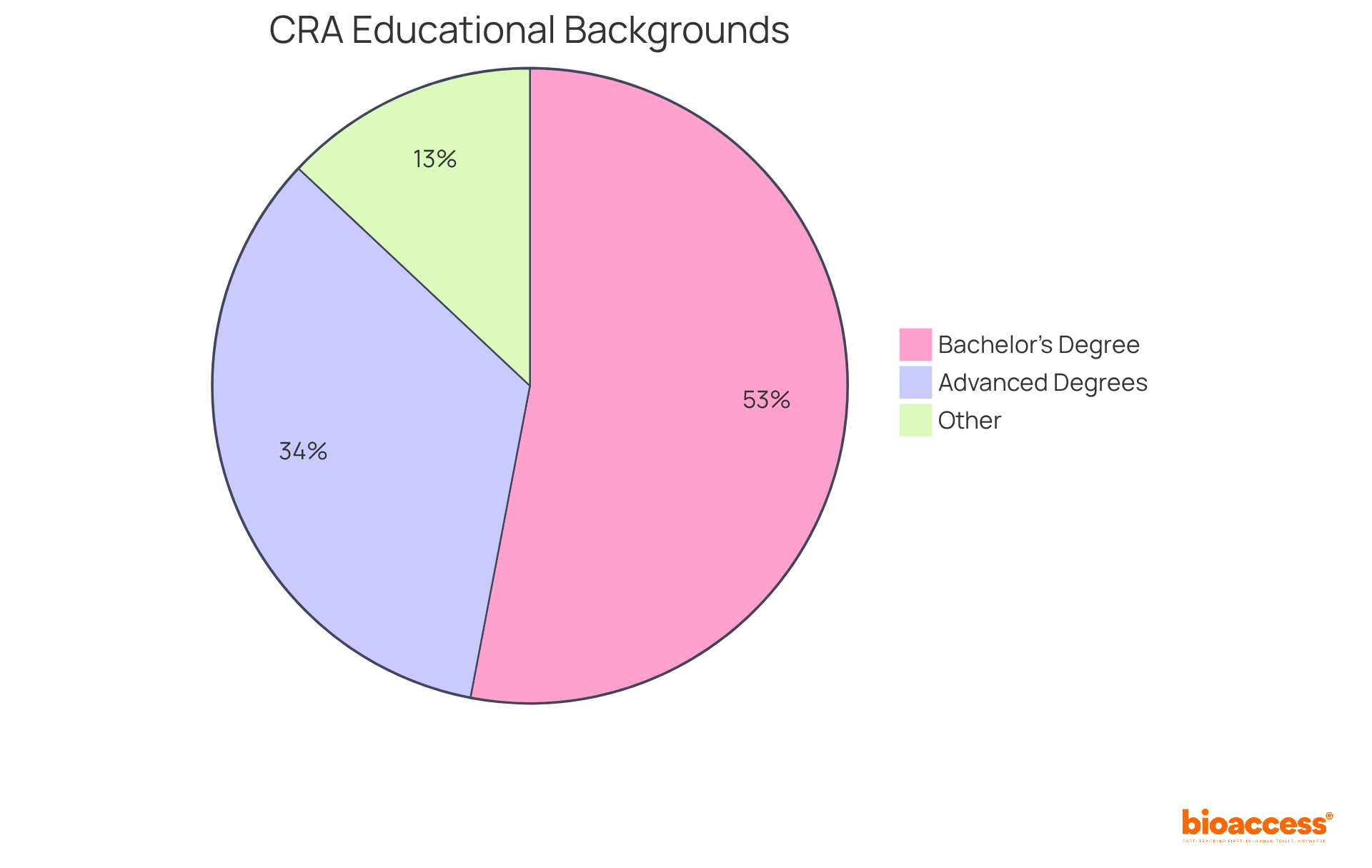
Educational Background: Most CRAs possess a bachelor’s degree in life sciences, nursing, pharmacy, or a related field. Notably, approximately 53% of CRAs hold a bachelor’s degree, while over 34% have advanced degrees, significantly enhancing job prospects and earning potential. In fact, professionals with master's degrees earn 93% higher average salaries compared to those with undergraduate qualifications, making advanced education a valuable investment.
Certifications: Pursuing certifications can greatly benefit your career trajectory. Key certifications include:
The medical research sector is expected to attain $52.0 billion by 2026, highlighting the increasing need for Clinical Research Associates and the significance of certifications in a competitive market. Moreover, over 40% of interns in medical research transition into permanent positions after their internships, emphasizing the possible routes for aspiring CRAs. Investing in these educational and certification pathways will equip you with the knowledge and credentials necessary to understand how to become a clinical research associate and excel in the competitive area of research in healthcare.
Gaining practical experience is a critical step in learning how to become a clinical research associate (CRA). To navigate this path effectively, consider the following strategies:
By actively pursuing these experiences, you will establish a robust foundation for understanding how to become a clinical research associate. The medical research market is projected to surpass $80 billion by 2025, underscoring the growing demand for skilled professionals in this field. As one expert noted, "research professionals in the medical field are in high demand with strong competition among employers.
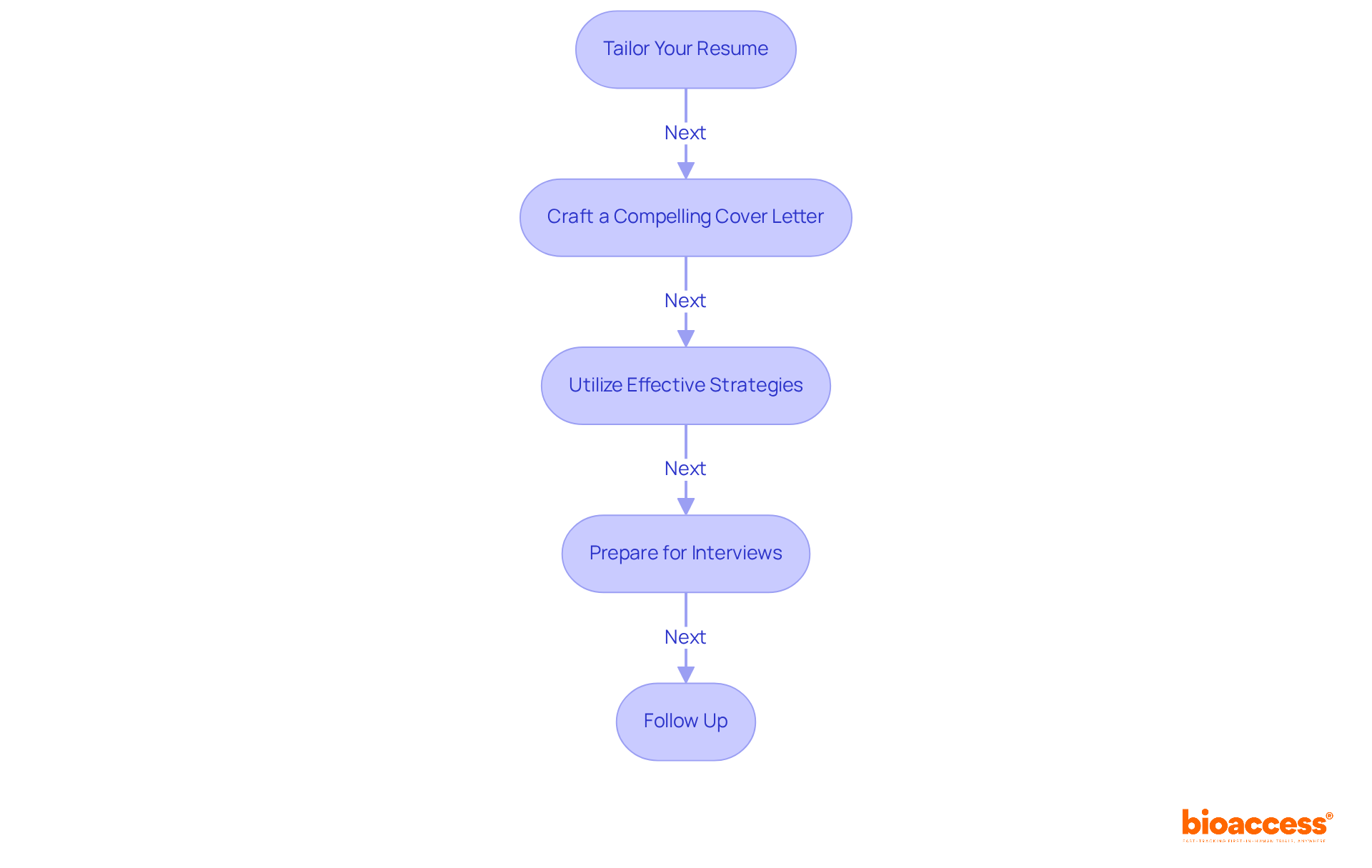
Navigating the job application process is a critical step toward learning how to become a clinical research associate (CRA). With the right approach, you can enhance your application significantly. Follow these essential steps:
Tailor Your Resume: Highlight your relevant education, certifications, and practical experience. Utilize keywords from the job description to align your resume with the employer's needs. Resumes that closely match job titles can increase your chances of landing an interview by 3.5 times.
Craft a Compelling Cover Letter: Your cover letter should effectively convey your enthusiasm for medical research and clarify why you are a fitting candidate for the role. Personalization is crucial; address it to a specific person if possible and include relevant examples that demonstrate your qualifications. Including a cover letter can make you 1.9 times more likely to be invited for an interview.
Utilize Effective Strategies: Incorporate quantifiable achievements in your cover letter to showcase your impact. For instance, mention specific projects you contributed to and the outcomes achieved. This approach can significantly enhance your appeal to hiring managers, as 34% consider the absence of quantifiable achievements a deal-breaker.
Prepare for Interviews: Research common interview questions for CRAs and practice your responses. Be ready to discuss your experience, problem-solving abilities, and knowledge of trial processes. Demonstrating your knowledge and enthusiasm can set you apart from other candidates.
Follow Up: After interviews, send a thank-you email to express your appreciation for the opportunity and reiterate your interest in the position. This simple gesture can reinforce your enthusiasm and keep you top of mind for hiring managers.
By diligently following these steps, you will enhance your chances of successfully understanding how to become a clinical research associate and advancing your career in clinical research.
Becoming a Clinical Research Associate (CRA) is a rewarding journey that necessitates a clear understanding of the role, educational background, practical experience, and strategic job application techniques. This guide outlines the essential steps to embark on this career path, emphasizing the significance of each aspect in ensuring success within the dynamic field of clinical research.
Key points discussed encompass the multifaceted responsibilities of CRAs, including:
Furthermore, a solid educational foundation, encompassing relevant degrees and certifications, is highlighted as a crucial factor in enhancing job prospects. Gaining practical experience through internships and entry-level positions is equally emphasized, as these opportunities provide invaluable insights into the clinical trial process while helping to build a professional network.
Ultimately, the demand for Clinical Research Associates is on the rise, presenting a wealth of opportunities for those willing to invest in their education and experience. Aspiring CRAs are encouraged to actively pursue internships, tailor their job applications, and continuously seek professional development to distinguish themselves in a competitive market. By following these steps, individuals can position themselves for a successful and fulfilling career in clinical research, contributing to advancements in healthcare and improved patient outcomes.
What is the role of a Clinical Research Associate (CRA)?
A Clinical Research Associate (CRA) serves as a vital link between sponsors and research sites, playing a crucial role in the research process.
What are the key responsibilities of a CRA?
The key responsibilities of a CRA include monitoring clinical trials, managing research sites, collecting and verifying data, maintaining communication with stakeholders, and reporting on study progress and issues.
How do CRAs ensure compliance during clinical trials?
CRAs ensure compliance by monitoring trials to adhere to protocols, regulatory requirements, and Good Clinical Practice (GCP) guidelines, which are essential for maintaining the integrity of the research.
What does site management involve for a CRA?
Site management involves selecting and overseeing clinical research locations, ensuring they are equipped with necessary resources and trained personnel, performing feasibility studies, and selecting primary investigators (PIs).
Why is data collection and verification important for CRAs?
Data collection and verification are important because CRAs guarantee the accuracy and integrity of the data, which are vital for the success of the study.
What role does communication play in a CRA's responsibilities?
Communication is essential for CRAs to maintain open lines with investigators, sponsors, and regulatory bodies, facilitating smooth operations and addressing challenges that may arise during the study.
What types of reports do CRAs prepare?
CRAs prepare comprehensive documents detailing study progress, encountered issues, study status, inventory, and adverse events, ensuring transparency and providing valuable insights to stakeholders.
What is the projected demand for Clinical Research Associates in the future?
The demand for Clinical Research Associates is projected to increase significantly by 2025, with the clinical trial market expected to surpass 70 billion dollars.