


The article emphasizes the critical process of selecting an independent investigator for clinical trials, highlighting the essential qualifications, experience, regulatory knowledge, and network resources required. It outlines specific criteria that are indispensable for ensuring the integrity and success of clinical studies. These criteria include:
Each of these elements plays a pivotal role in fostering trust and efficacy in clinical research, ultimately contributing to the advancement of medical knowledge.
Selecting the right independent investigator for clinical trials is a pivotal decision that can significantly influence the success of medical research. These professionals not only ensure adherence to regulatory standards and ethical practices but also play a crucial role in participant recruitment and data integrity.
However, with the increasing complexities of clinical studies and the evolving landscape of regulatory requirements, organizations must identify candidates who possess both the necessary qualifications and the strategic networks to enhance trial outcomes.
This article delves into the essential criteria for evaluating independent investigators, offering insights into their qualifications, experience, and the resources they bring to the table.
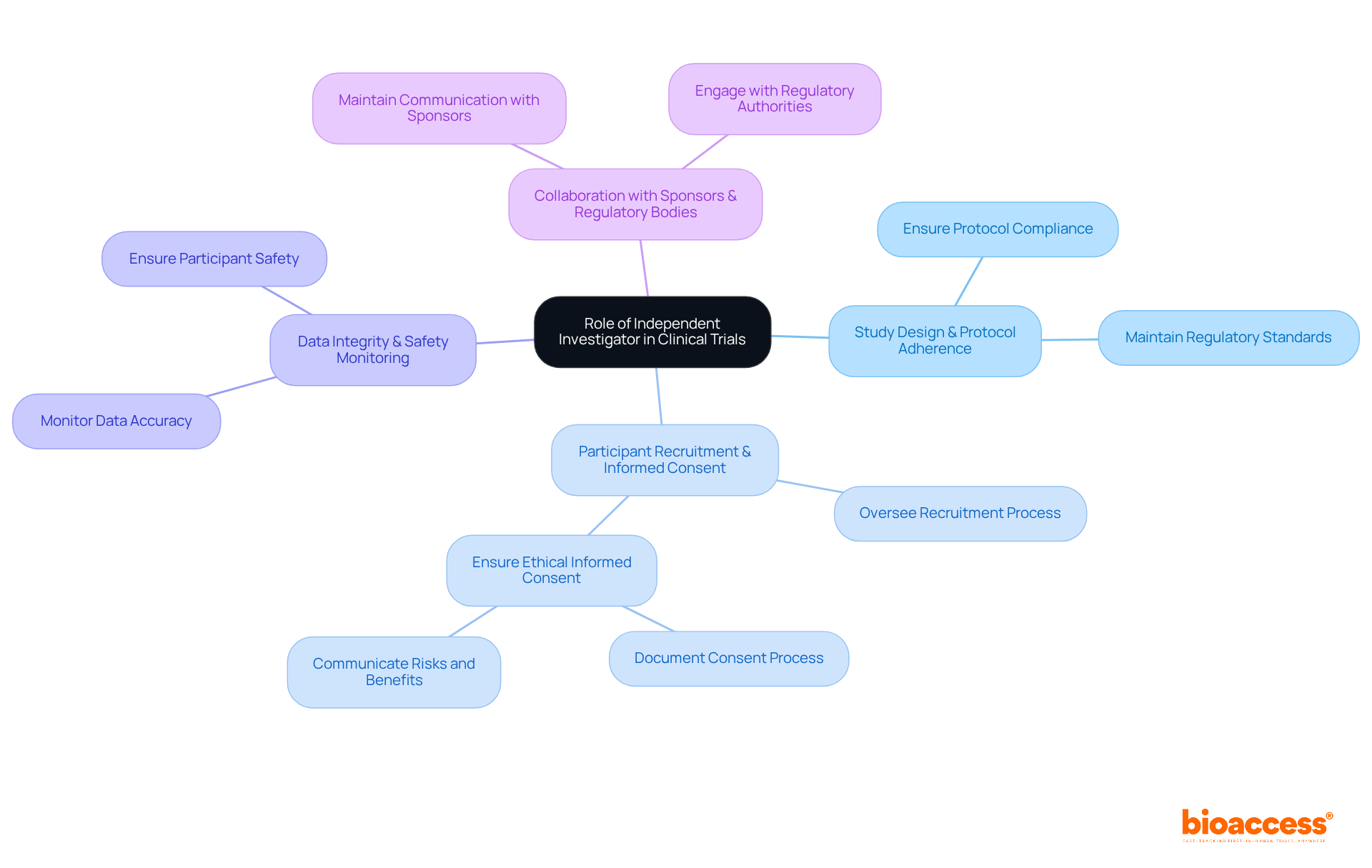
An independent investigator plays a crucial role in clinical studies, serving as the primary individual accountable for the implementation of the study at a designated site. This position is vital for ensuring the integrity and success of medical research. Their key responsibilities encompass several critical areas:
Recognizing these roles is essential for identifying candidates with the requisite skills and experience to manage the complexities of medical studies effectively. The Belmont Report underscores the importance of ethical behavior in research, emphasizing that researchers must guarantee fair treatment and informed decisions for participants. By adhering to these principles, the work of independent investigators significantly contributes to the effectiveness and trustworthiness of medical studies.
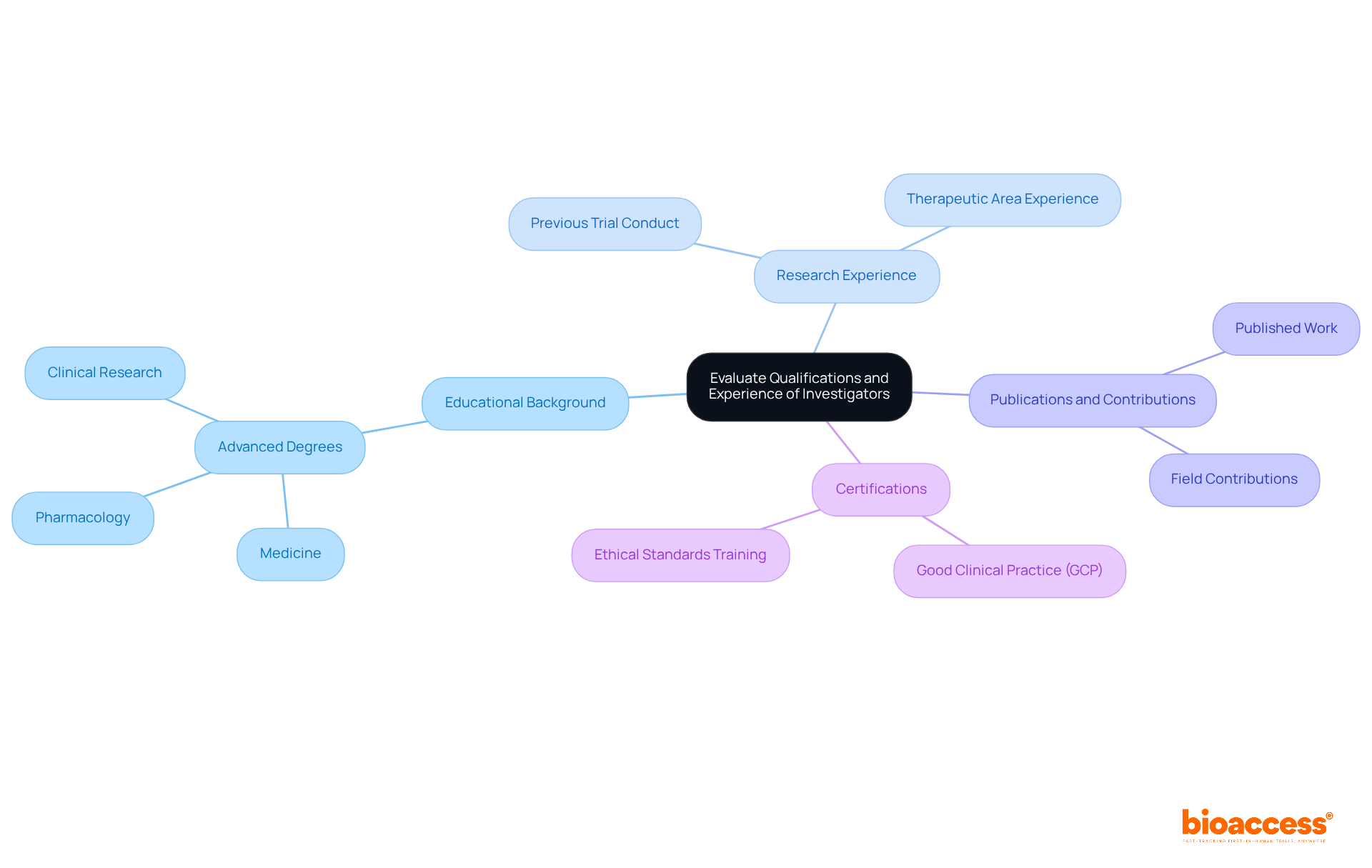
When evaluating potential investigators for clinical trials, it is essential to consider several key qualifications and experiences:
Considering the recent patterns showing a decrease in the number of consistently involved research professionals, it is becoming increasingly crucial to prioritize candidates with significant experience. The percentage of researchers participating in just one study has increased, emphasizing the necessity for ongoing involvement in medical research. By carefully assessing these factors, you can pinpoint candidates who are not only qualified but also skilled at handling the complexities inherent in medical studies.
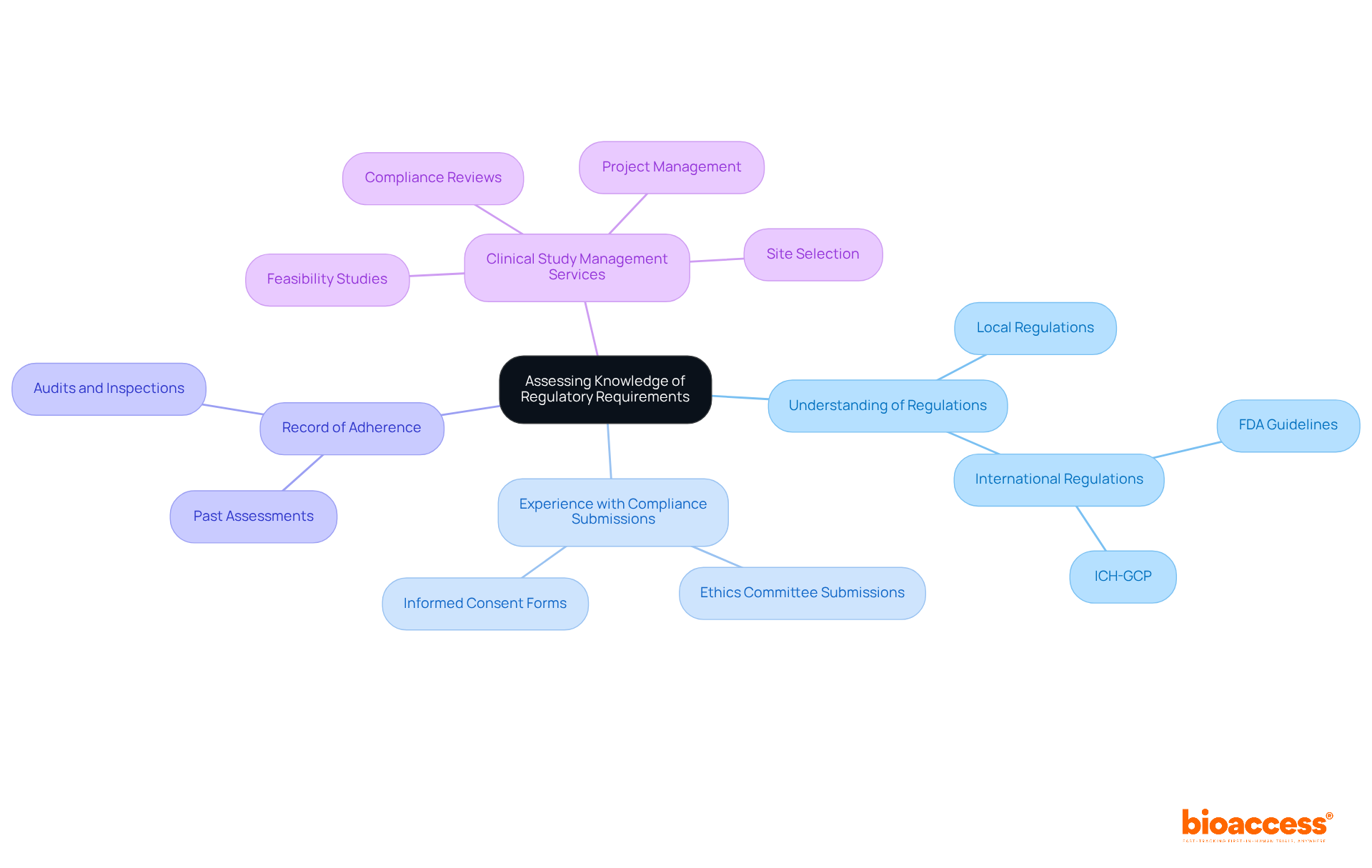
To ensure compliance with regulatory requirements, it is essential to assess the following factors:
Understanding of Local and International Regulations: Investigators must possess a comprehensive understanding of regulations such as FDA guidelines, ICH-GCP, and local regulatory requirements pertinent to the trial's location. Familiarity with these regulations is crucial for ethical practices and protecting the rights of human subjects in clinical trials.
Experience with Compliance Submissions: Assess the researcher's experience in preparing and submitting compliance documents, including ethics committee submissions and informed consent forms. A solid foundation in these areas suggests their ability to manage the intricacies of compliance effectively.
Record of Adherence: Examine the researcher's background of adherence in past assessments, including any audits or inspections carried out by governing entities. A proven track record of compliance with regulatory standards not only enhances the reliability of study outcomes but also fosters trust among stakeholders.
In early 2025, the FDA is expected to implement guidance that mandates a single Institutional Review Board (IRB) for multicenter studies, streamlining the ethical review process for site networks managing multiple locations. This guidance aims to reduce duplication and standardize requirements for sponsors, CROs, and research sites, enabling more efficient coordination across locations.
Furthermore, utilizing extensive clinical study management services, like those provided by bioaccess, can greatly improve the researcher's capacity to oversee compliance during the study. These services encompass feasibility studies, site selection, compliance reviews, testing setup, import permits, project management, and reporting. The knowledge of specialists such as Ana Criado, who has experience in regulatory affairs and biomedical engineering, along with Katherine Ruiz, a professional in regulatory affairs for medical devices, can further assist researchers in navigating these complexities. A comprehensive evaluation of these factors will guarantee that the researcher can efficiently handle compliance during the study, ultimately aiding in the integrity and success of the clinical examination.
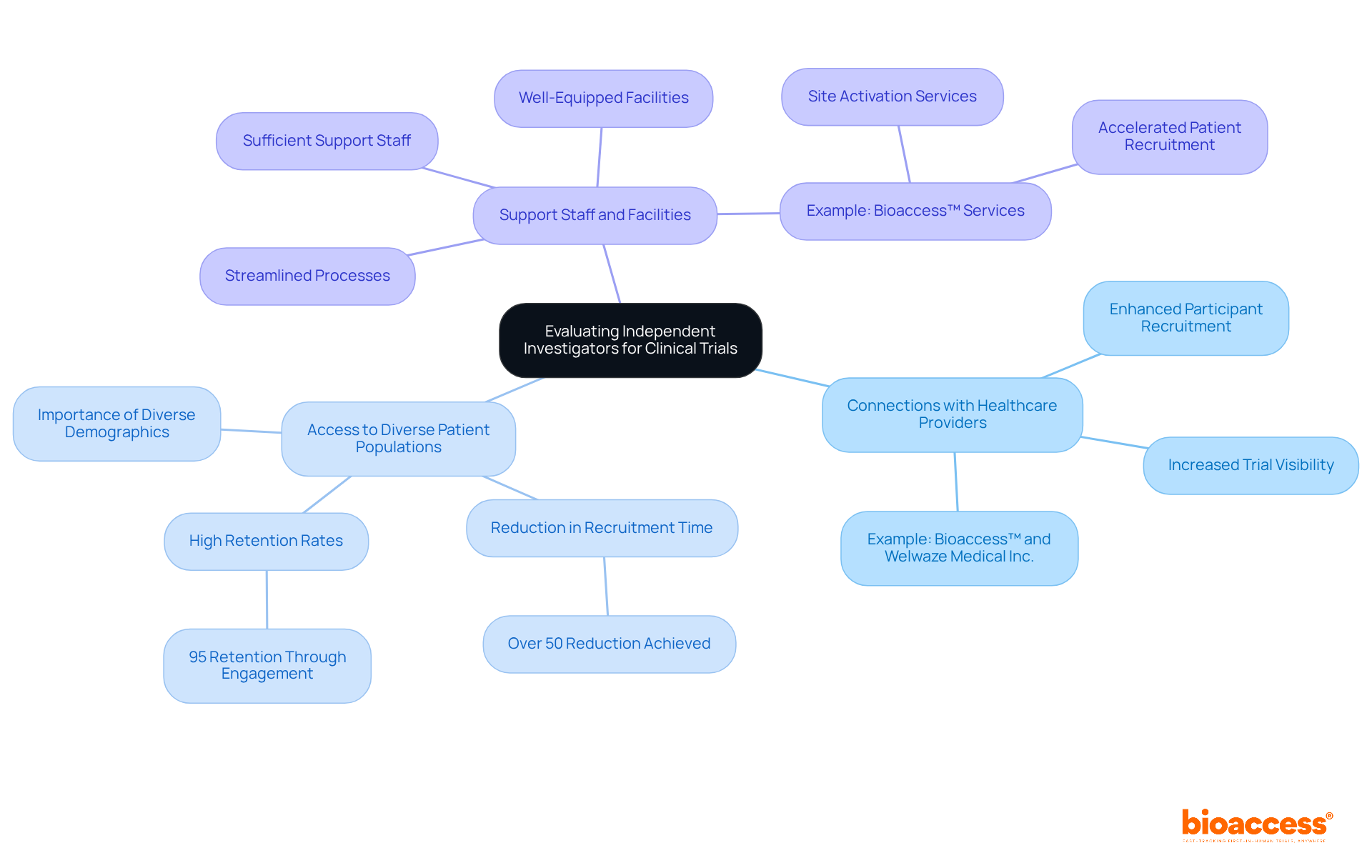
When evaluating the network and resources of an independent investigator, it is crucial to consider several key factors that directly impact the success of clinical trials.
Connections with Healthcare Providers: Investigators who cultivate robust relationships with local healthcare providers can significantly enhance participant recruitment and elevate the trial's visibility. Such connections foster higher trust levels among potential participants, which is essential for enrollment success. For instance, Bioaccess™ has effectively collaborated with Welwaze Medical Inc. to launch the Celbrea® medical device in Colombia, illustrating how strategic partnerships can facilitate access to healthcare networks and improve recruitment outcomes.
Access to Diverse Patient Populations: Assessing the researcher's capability to reach diverse patient demographics is paramount. Trials requiring specific populations benefit from researchers who can tap into varied patient networks, ensuring a representative sample that aligns with study criteria. Bioaccess™ has demonstrated its proficiency in enhancing clinical study services, achieving over a 50% reduction in recruitment time and 95% retention rates through effective patient engagement strategies.
Support Staff and Facilities: It is essential to evaluate whether the investigator has access to sufficient support staff and facilities. A well-equipped team and infrastructure are vital for conducting experiments efficiently, as they streamline processes and enhance overall management. Bioaccess™ provides accelerated patient recruitment and site activation services, addressing common challenges faced by medical device startups, including regulatory hurdles and financial constraints.
By thoroughly evaluating these factors, you can select an independent investigator who not only possesses the requisite qualifications but also has the resources and connections to facilitate the successful execution of the research study. This strategic approach can lead to improved patient recruitment success rates, as evidenced by networks that effectively leverage healthcare provider relationships and diverse patient access. Furthermore, considering a four-part framework encompassing strategy, science, support, and creative elements can further expedite patient-centric clinical trials.
Selecting an independent investigator for clinical trials stands as a pivotal decision that can greatly influence the success of medical research. These investigators bear essential responsibilities, including study design, participant recruitment, data integrity, and adherence to regulatory standards. A thorough understanding of their multifaceted duties is crucial for pinpointing candidates who possess the requisite expertise and ethical commitment to navigate the complexities inherent in clinical trials.
When evaluating potential investigators, several key factors warrant consideration:
Investigators must not only hold advanced degrees and maintain a solid publication history but also exhibit a proven capacity to manage compliance and ensure participant safety. Furthermore, robust networking capabilities and access to diverse patient populations can significantly bolster recruitment efforts, ultimately resulting in more substantial study outcomes.
In light of these insights, it becomes imperative for stakeholders in clinical research to adopt a strategic approach when selecting independent investigators. By prioritizing candidates who demonstrate a blend of qualifications, experience, and resources, the integrity and success of clinical trials can be markedly enhanced. This meticulous selection process not only contributes to the advancement of medical knowledge but also guarantees that the rights and well-being of participants are protected throughout the research journey.
What is the role of an independent investigator in clinical trials?
An independent investigator is responsible for the implementation of a clinical study at a designated site, ensuring the integrity and success of medical research.
What are the key responsibilities of an independent investigator?
Their key responsibilities include ensuring study design and protocol adherence, overseeing participant recruitment and informed consent, monitoring data integrity and participant safety, and collaborating with sponsors and regulatory bodies.
How does an independent investigator ensure study design and protocol adherence?
They ensure that the study adheres to the approved protocol and meets all regulatory requirements, which is essential for maintaining the integrity of the trial.
What is involved in participant recruitment and informed consent?
The independent investigator oversees participant recruitment and ensures that informed consent is obtained ethically and transparently, thus protecting the rights of participants.
Why is data integrity and safety monitoring important in clinical trials?
Ensuring data integrity and monitoring participant safety throughout the study is vital for ethical research conduct and helps maintain the trustworthiness of the study's findings.
How do independent investigators collaborate with sponsors and regulatory bodies?
They maintain open communication with sponsors, regulatory authorities, and ethics committees to ensure compliance and address any arising issues, fostering a collaborative environment.
What ethical principles guide the work of independent investigators?
The Belmont Report emphasizes the importance of ethical behavior in research, requiring researchers to guarantee fair treatment and informed decisions for participants, which independent investigators uphold in their work.