


Integrating ISO 14971:2019 into medical device development is crucial for effectively managing risks and ensuring patient safety throughout the product lifecycle. This structured approach encompasses:
Collectively, these elements not only enhance compliance with regulatory standards but also significantly improve overall device quality. By prioritizing these practices, organizations can foster a culture of safety and reliability in their products.
Navigating the complexities of medical device development requires a robust framework for managing risks, and ISO 14971:2019 is pivotal in this endeavor. This standard not only facilitates the identification and mitigation of potential hazards but also ensures compliance with regulatory demands, thereby enhancing patient safety and product quality.
Nevertheless, integrating ISO 14971:2019 into existing development processes often presents significant challenges. How can developers effectively adopt this standard to not only ensure compliance but also drive innovation in a rapidly evolving medical landscape?
ISO 14971:2019 is fundamental for managing hazards in medical products, offering a structured framework for identifying, assessing, and controlling risks throughout the product lifecycle. The ISO 14971:2019 standard is crucial for developers, as it facilitates early hazard identification and effective risk mitigation, thereby ensuring compliance with regulatory standards. By advocating for a systematic approach, the standard ISO 14971:2019 enhances patient safety and elevates the overall quality of medical devices.
Key principles include:
Real-world applications of these principles underscore their efficacy in minimizing risks and enhancing patient outcomes. As the medical device landscape continues to evolve, understanding and applying ISO 14971:2019 is essential for fostering innovation while safeguarding public health.

To effectively integrate ISO 14971:2019 into your development process, consider the following steps:
Form a Risk Mitigation Team: Gather a devoted group with members from engineering, quality assurance, regulatory affairs, and other pertinent fields to guarantee a thorough approach to managing uncertainties. As Etienne Nichols highlights, having set up safety oversight systems that adhere to ISO 14971:2019 is essential for successful product development.
Define the Scope: Clearly articulate the extent of your oversight activities, including the intended use of the medical instrument, the target user population, and any applicable regulatory requirements.
Formulate a Risk Mitigation Strategy: Create a comprehensive strategy for addressing uncertainties that details procedures, responsibilities, and timelines for associated activities throughout the product's lifecycle, ensuring alignment with the standards of ISO 14971:2019. This plan acts as the basis for your management process, as emphasized in the case study 'Building a Strong Management Foundation.'
Identify Hazards: Utilize brainstorming sessions and historical data analysis to systematically identify potential dangers associated with the device, ensuring a thorough understanding of threats.
Evaluate Hazards: Assess identified dangers by evaluating their severity and likelihood of occurrence, prioritizing them for further action based on established criteria.
Implement Control Measures: Develop and execute strategies to reduce identified hazards, ensuring that all control measures are documented and communicated effectively to all stakeholders involved. Verification of these measures is essential for compliance with ISO 14971:2019.
Examine and Revise: Regularly examine and revise the safety plan and related documentation to reflect any changes in the device, its intended use, or regulatory requirements. This repetitive aspect of overseeing potential issues is essential for sustaining continuous adherence and adjusting to new data.

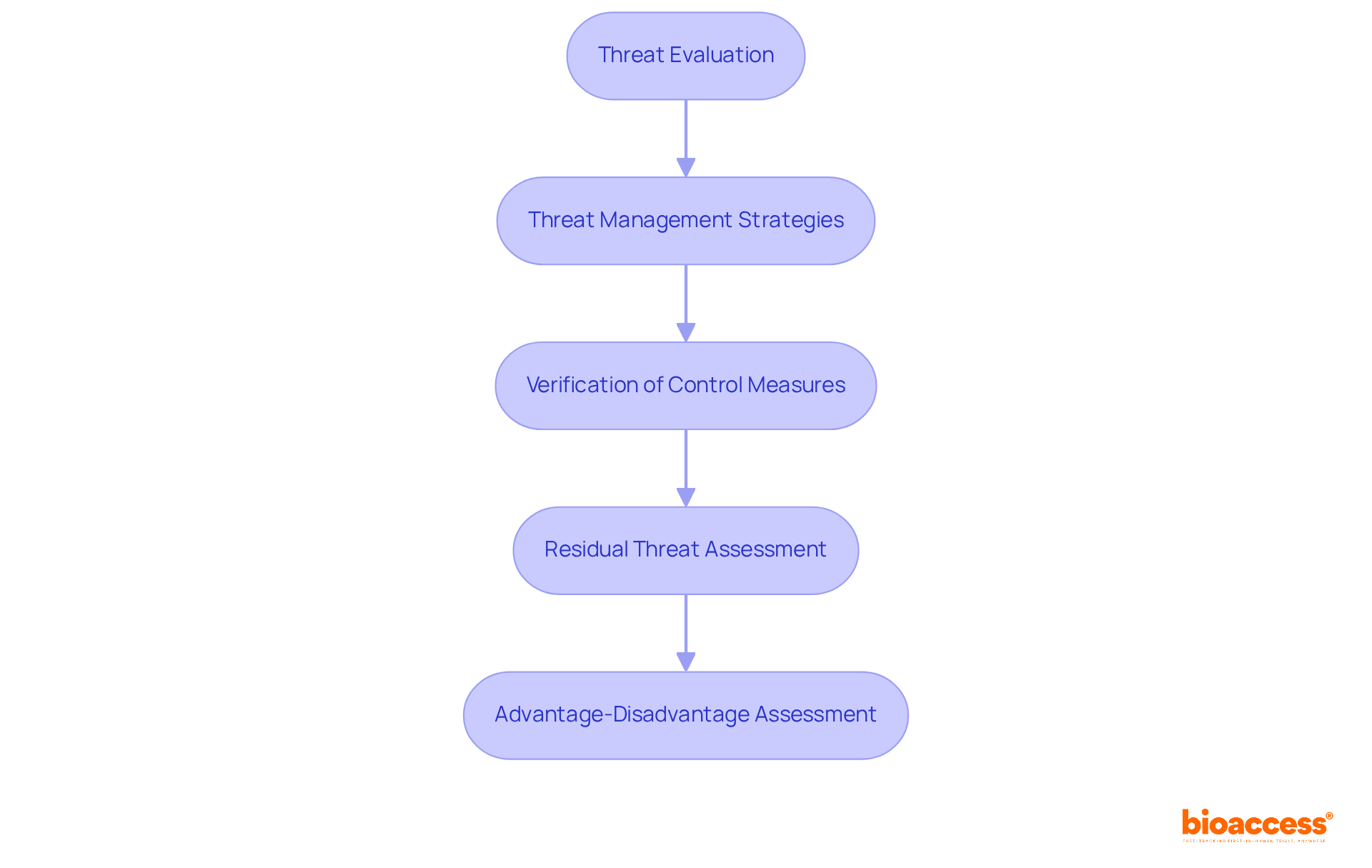
To conduct comprehensive risk management, adhere to the following steps:
Threat Evaluation: Utilize both qualitative and quantitative techniques to assess the dangers associated with each identified hazard. Consider critical factors such as the severity of harm, probability of occurrence, and detectability.
Threat Management Strategies: For every recognized threat, determine appropriate control measures. These may encompass design modifications, protective measures, or clear warnings and instructions for use.
Verification of Control Measures: Confirm the effectiveness of the implemented control measures through rigorous testing and evaluations. Meticulously document the results to demonstrate compliance with iso 14971:2019.
Residual Threat Assessment: Following the application of control measures, evaluate any remaining residual threats. Assess whether these hazards are acceptable given the equipment's intended application and the prevailing regulatory standards.
Advantage-Disadvantage Assessment: Conduct an advantage-disadvantage assessment to verify that the benefits of the medical device outweigh the remaining hazards. This analysis should be thoroughly documented and subjected to regular review.
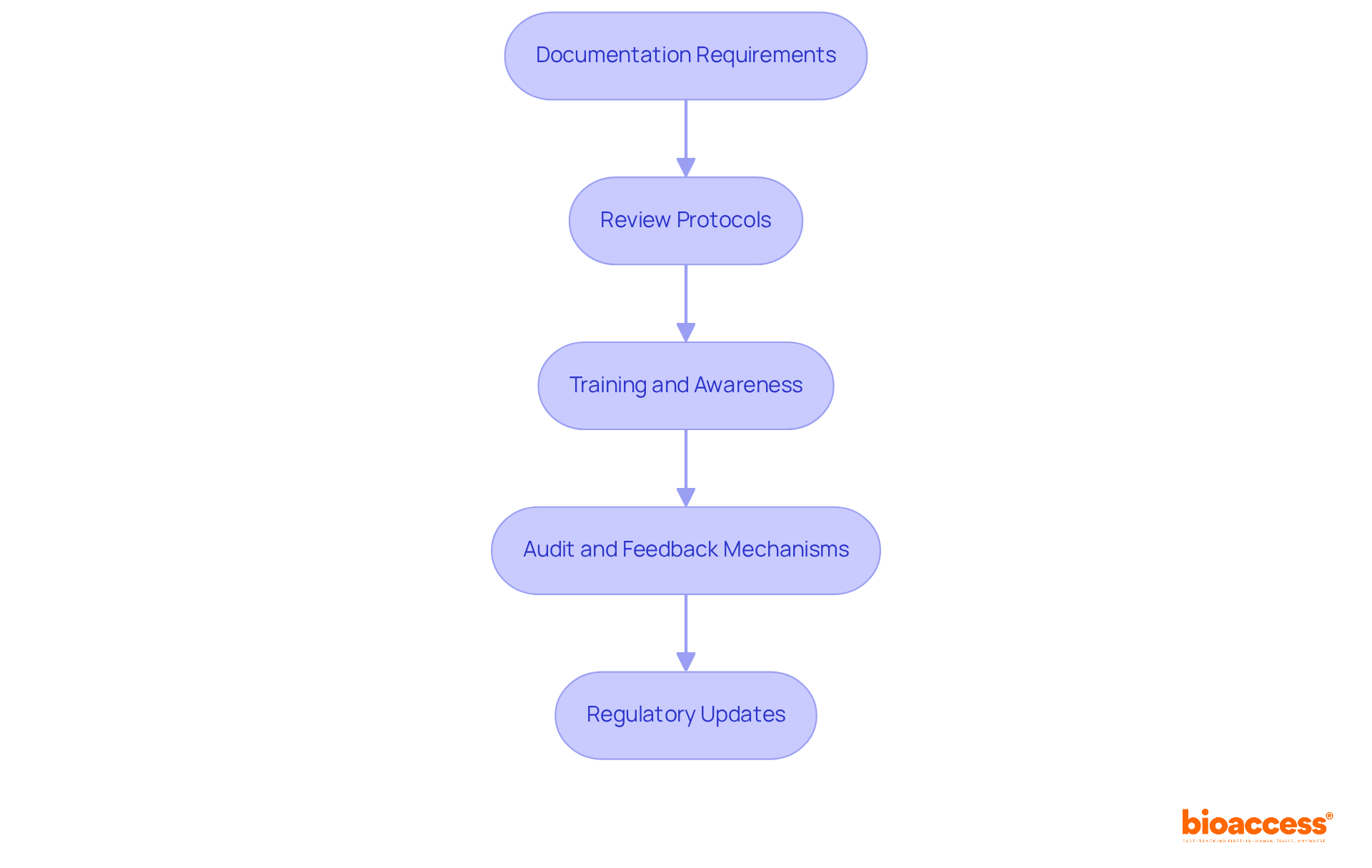
To establish effective documentation and review protocols for risk management, it is essential to consider the following steps:
Documentation Requirements: Clearly identify and record all essential hazard evaluation activities, including assessments, control measures, and verification results. Documentation must be concise, accessible, and organized to facilitate easy reference. Moreover, producers are required to maintain a comprehensive file for hazard control that gathers all documents from the hazard evaluation procedure, ensuring adherence to established protocols.
Review Protocols: Develop an organized timetable for routine evaluations of oversight documentation and procedures. This should encompass regular assessments of the effectiveness of control measures and prompt updates to the mitigation plan as necessary, ensuring that all elements of threat assessment are consistently reviewed.
Training and Awareness: It is imperative that all team members involved in the development procedure receive thorough instruction on ISO 14971:2019 and the specific safety protocols of the organization. Fostering an environment of safety and adherence within the team is crucial for effective hazard control.
Audit and Feedback Mechanisms: Implement systematic review methods to evaluate adherence to the standards outlined in ISO 14971:2019 and to gather constructive feedback from stakeholders. Utilize this information to drive ongoing enhancements in the hazard oversight system, ensuring it remains efficient and relevant.
Regulatory Updates: Maintain vigilance regarding changes in regulations and standards related to risk management in medical devices. Regularly update your documentation and processes to ensure ongoing compliance and alignment with current regulatory expectations.
Integrating ISO 14971:2019 into medical device development is crucial for effective risk management and enhancing patient safety. This standard offers a comprehensive framework for identifying, assessing, and controlling risks throughout the product lifecycle, making it indispensable for developers who aim to achieve regulatory compliance while fostering innovation in healthcare.
The article presents a structured approach to integrating ISO 14971:2019, underscoring the formation of a dedicated risk mitigation team, defining the scope of oversight activities, and implementing a robust risk management strategy. It emphasizes the necessity of documenting hazard evaluations, conducting thorough assessments of identified threats, and regularly reviewing protocols to maintain compliance. By adhering to these steps, developers can systematically manage risks, ensuring that safety measures are both effective and well-documented.
Ultimately, the successful integration of ISO 14971:2019 not only safeguards public health but also promotes the continuous advancement of medical technology. Embracing these practices transcends mere compliance; it fosters a culture of safety and excellence in medical device development. Stakeholders are urged to prioritize these standards to enhance product quality and, most importantly, to protect the patients who depend on them.
What is ISO 14971:2019 and why is it important in medical device development?
ISO 14971:2019 is a standard for managing hazards in medical products, providing a structured framework for identifying, assessing, and controlling risks throughout the product lifecycle. It is important because it facilitates early hazard identification and effective risk mitigation, ensuring compliance with regulatory standards and enhancing patient safety.
What are the key principles of ISO 14971:2019?
The key principles include integrating uncertainty management into the design process, documenting safety-related characteristics, and analyzing reasonably foreseeable misuse.
How does ISO 14971:2019 contribute to patient safety?
By advocating for a systematic approach to risk management, ISO 14971:2019 enhances patient safety and improves the overall quality of medical devices.
What is the significance of documenting safety-related characteristics in ISO 14971:2019?
Documenting safety-related characteristics is critical as it ensures that all safety aspects are considered and managed throughout the development process, contributing to the overall safety and effectiveness of the medical device.
How does ISO 14971:2019 address the issue of misuse of medical devices?
The standard obligates developers to analyze reasonably foreseeable misuse, which helps in identifying potential risks and implementing measures to mitigate them.
Why is understanding ISO 14971:2019 essential for medical device developers?
Understanding and applying ISO 14971:2019 is essential for fostering innovation in medical device development while safeguarding public health, especially as the medical device landscape continues to evolve.