


Integrating real-world data (RWD) into clinical trials significantly enhances the relevance and validity of research outcomes. By providing insights from diverse sources such as electronic health records, insurance claims, and patient registries, RWD integration addresses critical challenges in clinical research.
Effective RWD integration:
This approach underscores the importance of collaboration in advancing clinical research and improving patient outcomes.
Integrating Real World Data (RWD) into clinical trials is revolutionizing medical research, offering insights that mirror the realities of everyday patient experiences. This guide explores the significant advantages of RWD, emphasizing its role in enhancing the validity and relevance of clinical studies, while also addressing critical health disparities.
Nevertheless, the path to effective integration presents numerous challenges, ranging from concerns about data quality to navigating regulatory hurdles.
How can researchers adeptly maneuver these complexities to fully leverage the potential of RWD in their trials?
Real World Data (RWD) encompasses information gathered from a variety of sources beyond traditional clinical studies, including electronic health records (EHRs), insurance claims, and registries. Its significance in clinical research cannot be understated, as it offers critical insights into individual behaviors, treatment outcomes, and healthcare delivery within real-world settings.
By utilizing real world data integration clinical trials, researchers can markedly improve the external validity of their trials, ensuring that findings resonate more effectively with everyday clinical practice. This approach not only elucidates treatment effectiveness across diverse populations but also enhances care quality and informs regulatory decisions.
For example, bioaccess® has demonstrated its capability to enroll treatment-naive cardiology or neurology groups 50% faster than locations in the West, achieving substantial savings of $25K per individual with FDA-ready data—no rework, no delays. The patient-centric strategy employed by bioaccess has resulted in a more than 50% reduction in recruitment time, alongside an impressive retention rate exceeding 95%, underscoring how RWD can enhance study efficiency.
Furthermore, the incorporation of RWD can facilitate a 20-30% decrease in overall research costs, further highlighting its value in clinical research. Ultimately, real world data integration clinical trials not only bolster the validity of research but also address health disparities, paving the way for equitable advancements in care.
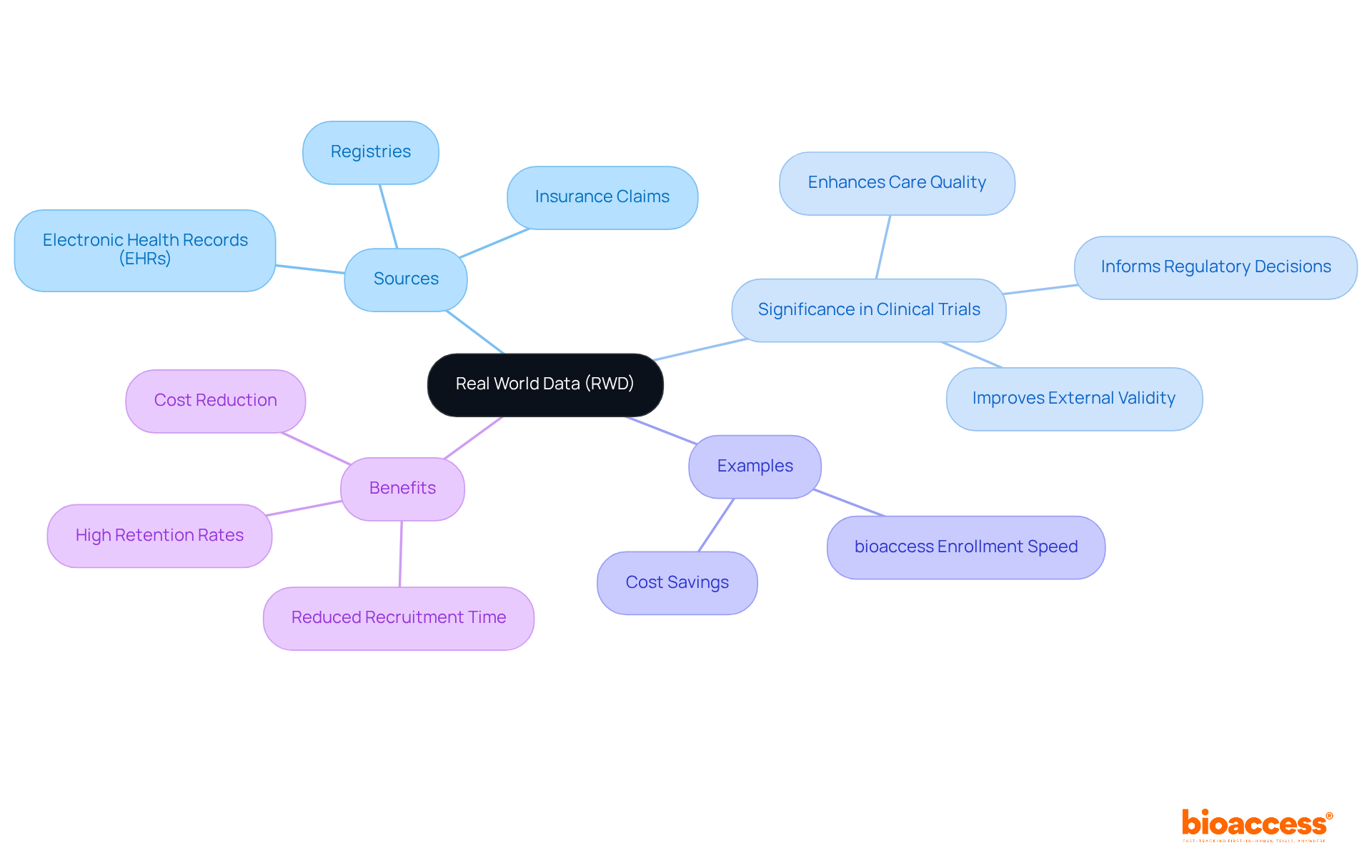
Integrating real world data integration clinical trials significantly enhances the quality and relevance of research outcomes. Key types of RWD include:
Electronic Health Records (EHRs): EHRs serve as a cornerstone of clinical research, containing comprehensive individual information such as demographics, diagnoses, medications, and treatment outcomes. As of 2021, over 95% of non-federal acute care hospitals in the U.S. had adopted certified EHR systems, reflecting their critical role in modern healthcare. Dr. Jennifer Priestley emphasizes that successful information analysis requires both technical skills and business acumen, highlighting the significance of EHRs in connecting these areas.
Claims Information: Insurance claims information offers valuable insights into healthcare utilization, costs, and outcomes, allowing researchers to analyze real-world treatment patterns and economic effects.
Participant Registries: These structured systems gather information about individuals with specific conditions, aiding long-term follow-up and outcome evaluation. They are essential for understanding disease progression and treatment effectiveness over time.
User-Generated Information: This category encompasses information from wearable devices, mobile health applications, and user surveys, providing real-time insights into individual health and behavior. Such information can improve client involvement and offer a more comprehensive perspective on treatment effects.
Social Media and Online Health Communities: These platforms can provide qualitative information on patient experiences and treatment perceptions, enhancing the understanding of patient-reported outcomes and satisfaction.
By comprehending these varied information types, researchers can choose the most pertinent sources for real world data integration clinical trials, ultimately resulting in more informed and significant research outcomes. Integrating these data types into real world data integration clinical trials not only aligns with the latest advancements in healthcare but also improves the overall quality of research.

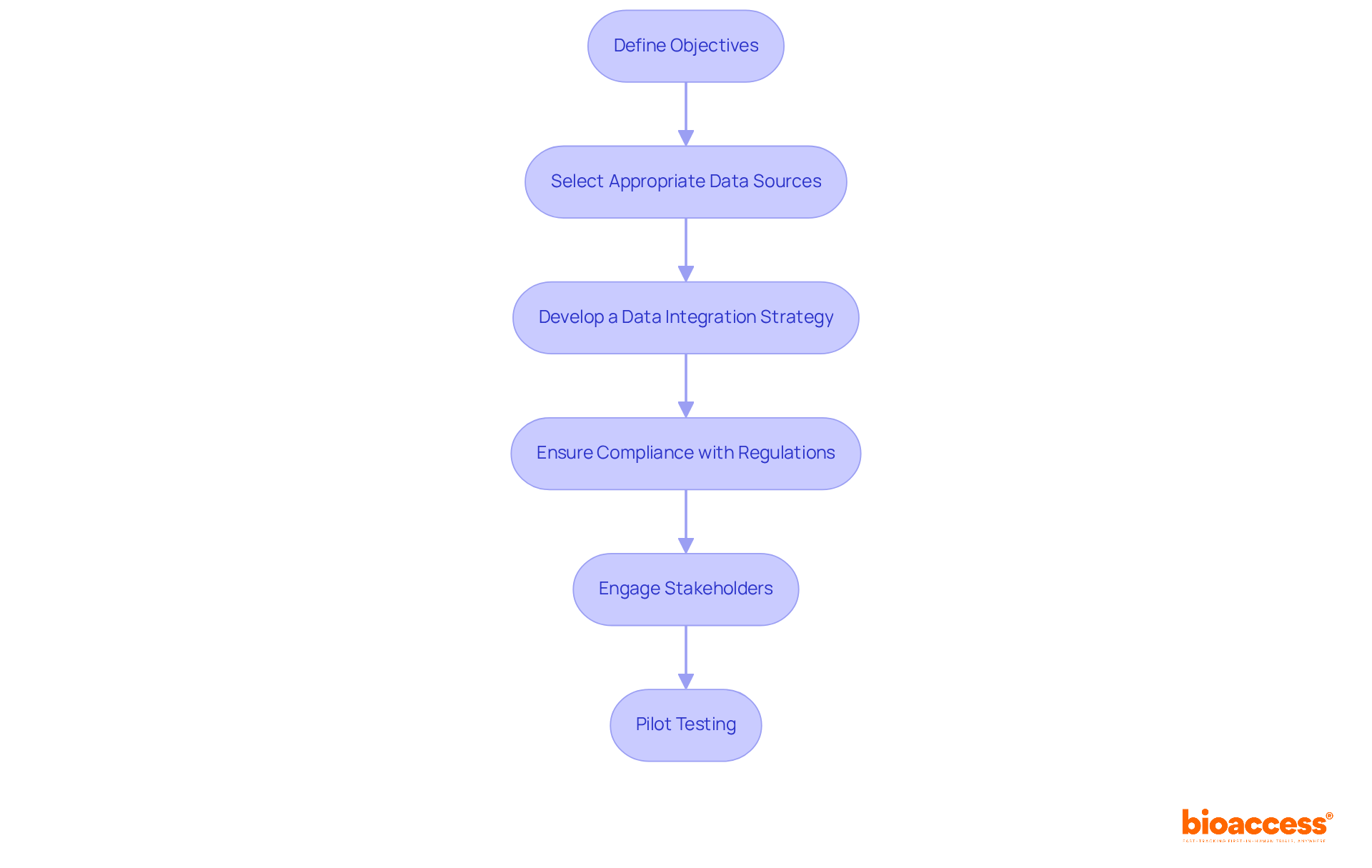
To effectively integrate Real World Data (RWD) into clinical trial protocols, consider the following strategies:
Define Objectives: Clearly outline the goals for using RWD in your study. Determine how it will enhance the study's design, patient recruitment, or outcome assessment. Expert views indicate that clearly outlined objectives are essential for enhancing the effect of RWD on study results.
Select Appropriate Data Sources: Choose RWD sources that align with your study objectives. Ensure that the information is relevant, reliable, and accessible. Statistics suggest that studies employing various RWD sources can improve patient representation and enhance the generalizability of findings.
Develop a Data Integration Strategy: Create a comprehensive plan that specifies how RWD will be gathered, processed, and examined alongside conventional clinical study information. Effective integration strategies have been demonstrated to enhance information management and boost the efficiency of operational processes. bioaccess offers comprehensive project management services that can assist in this planning phase, ensuring that all aspects of data integration are meticulously addressed.
Ensure Compliance with Regulations: Familiarize yourself with regulatory guidelines regarding the use of RWD in clinical studies. Following these standards is crucial for preserving the integrity of your study and ensuring regulatory approval of your findings. bioaccess provides expertise in compliance reviews and can help navigate the evolving regulatory frameworks, including those set by INVIMA, which oversees medical device classification and approval processes in Colombia.
Engage Stakeholders: Collaborate with key stakeholders, including regulatory bodies, ethics committees, and patient advocacy groups, to gain insights and support for your RWD integration efforts. Involving stakeholders early can promote smoother execution and improve the credibility of your study. Collaborative initiatives among pharmaceutical companies, regulatory agencies, and technology firms are essential for real world data integration clinical trials in healthcare.
Pilot Testing: Conduct pilot studies to evaluate the incorporation of RWD into your study protocols. This method assists in recognizing possible obstacles and improving your methodology prior to large-scale execution, ultimately resulting in more resilient experimental designs. RWD can streamline recruitment, leading to shorter timelines, making pilot testing a valuable step.
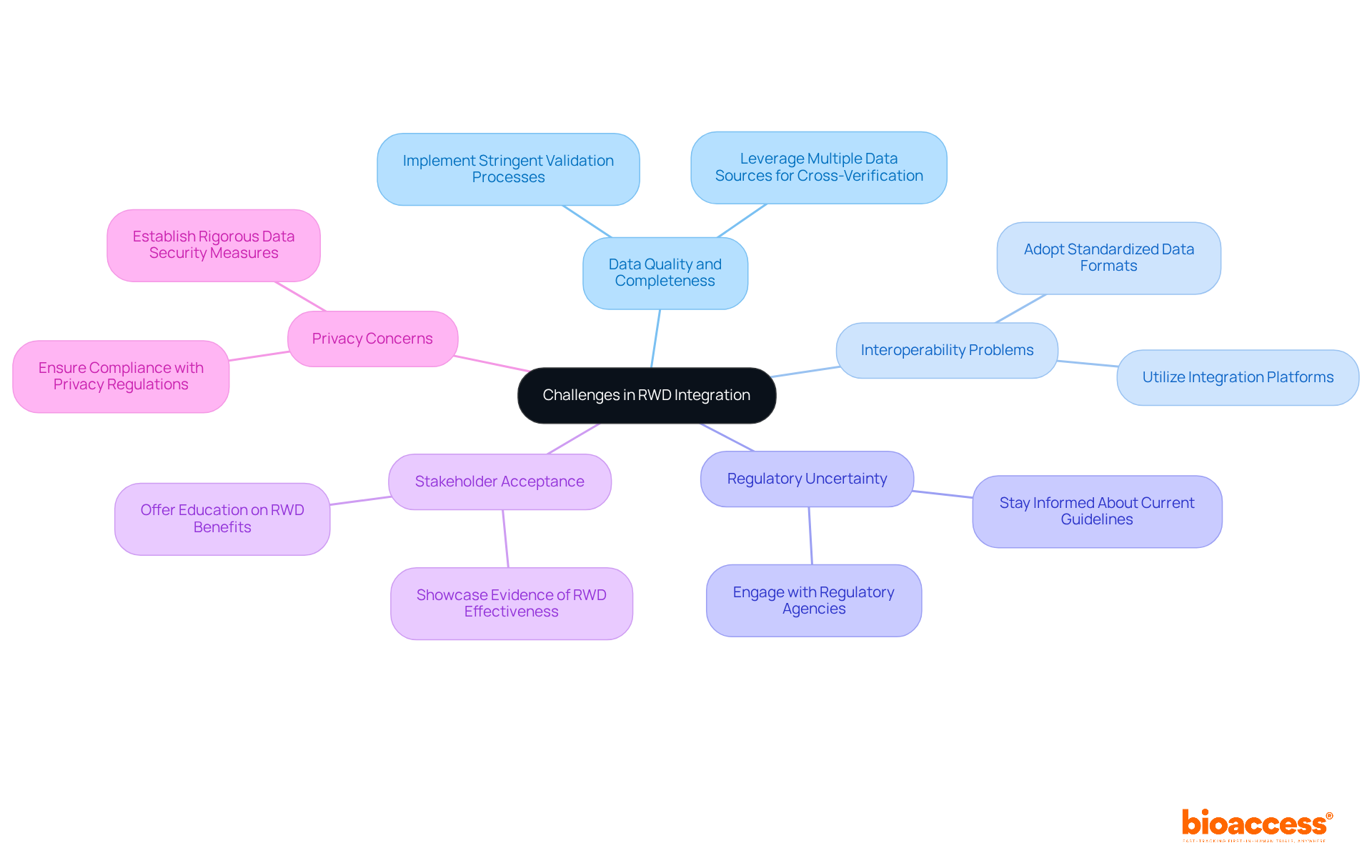
Integrating real world data integration clinical trials presents several challenges that require strategic solutions.
By proactively addressing these challenges, researchers can significantly enhance the effectiveness of real world data integration clinical trials.
Integrating Real World Data (RWD) into clinical trials represents a transformative approach that significantly enhances the relevance and validity of research outcomes. By effectively leveraging diverse data sources—such as electronic health records, claims information, and patient registries—researchers can obtain invaluable insights that accurately reflect actual patient experiences and treatment effectiveness within everyday healthcare settings. This integration not only improves the generalizability of findings but also plays a crucial role in addressing health disparities, ultimately leading to more equitable advancements in healthcare.
Throughout the article, we have discussed key strategies for successful RWD integration, including the importance of:
Furthermore, stakeholder engagement and pilot testing have been emphasized as essential steps to overcome challenges such as data quality, interoperability, and privacy concerns. By adopting these best practices, researchers can significantly enhance the efficiency and credibility of their clinical trials, paving the way for innovative solutions and improved patient outcomes.
The significance of integrating real world data in clinical research cannot be overstated. As the healthcare landscape continues to evolve, embracing RWD will not only streamline clinical trial processes but also foster a deeper understanding of treatment impacts across diverse populations. The call to action is clear: researchers, healthcare providers, and regulatory bodies must collaborate and invest in RWD strategies to unlock the full potential of clinical trials, ensuring that they reflect real-world scenarios and ultimately improve patient care.
What is Real World Data (RWD)?
Real World Data (RWD) refers to information collected from various sources outside traditional clinical studies, including electronic health records (EHRs), insurance claims, and registries.
Why is RWD important in clinical trials?
RWD is crucial in clinical research as it provides valuable insights into individual behaviors, treatment outcomes, and healthcare delivery in real-world settings, enhancing the external validity of trials.
How does RWD integration improve clinical trials?
By integrating RWD into clinical trials, researchers can ensure that findings are more relevant to everyday clinical practice, elucidate treatment effectiveness across diverse populations, and improve care quality.
What are some benefits of using RWD in clinical trials?
Benefits of using RWD include a significant reduction in recruitment time (over 50%), high retention rates (exceeding 95%), and a potential decrease in overall research costs by 20-30%.
Can you provide an example of RWD in action?
Bioaccess has shown the ability to enroll treatment-naive cardiology or neurology groups 50% faster than locations in the West, achieving savings of $25K per individual with FDA-ready data, demonstrating the efficiency of RWD integration.
How does RWD address health disparities?
The integration of RWD in clinical trials helps to address health disparities by ensuring that research findings are applicable to a broader range of populations, paving the way for equitable advancements in healthcare.