


Labeling translation in cosmetics is paramount, necessitating strict adherence to regulatory frameworks and clear communication of product identity. This is essential not only for compliance but also for fostering consumer trust. The significance of utilizing standardized terminology cannot be overstated; it ensures ingredient transparency and the provision of necessary hazard warnings. These practices are critical in avoiding legal issues while enhancing consumer safety and confidence.
Navigating the complex landscape of cosmetic labeling compliance presents a formidable challenge for businesses, particularly in light of evolving regulations and heightened consumer expectations. It is essential to understand the critical frameworks that govern product identification and safety, as these guidelines not only ensure legal adherence but also cultivate consumer trust.
How can companies effectively translate these regulations into clear, accurate labels that resonate with their target audience? This article explores key practices for labeling translation, providing insights into compliance, clarity, and consumer safety that every cosmetic producer should adopt.
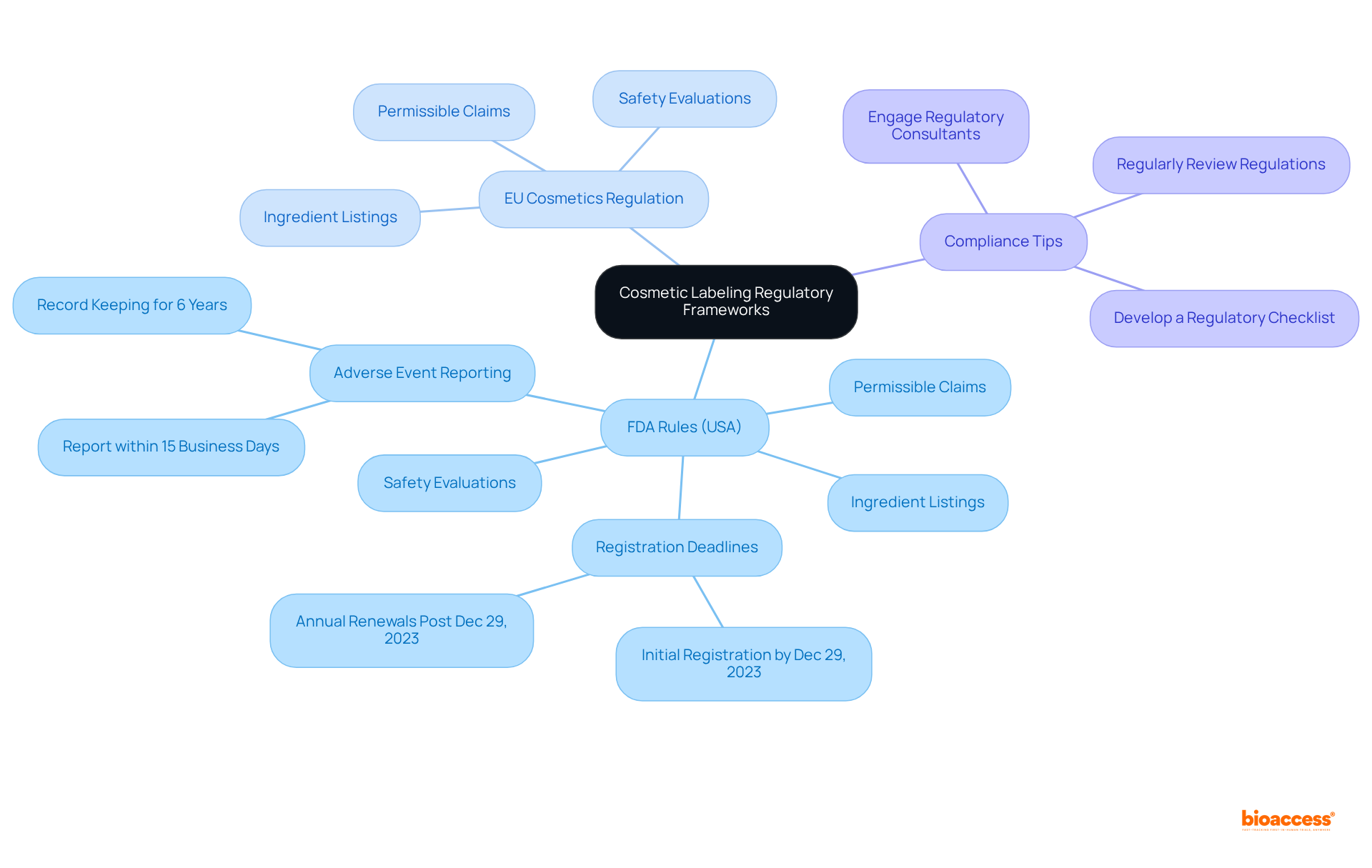
To ensure compliance with cosmetic requirements, it is essential to familiarize yourself with the relevant regulatory frameworks, such as the FDA rules in the United States and the EU Cosmetics Regulation in Europe. These regulations outline specific criteria for product identification, including permissible claims, ingredient listings, and safety evaluations. Notably, all businesses involved in the production or handling of cosmetic products must register with the FDA by December 29, 2023, highlighting the critical nature of compliance. It is advisable for companies to regularly review these regulations, as they are subject to change; staying informed is key to avoiding costly errors. Engaging with regulatory consultants or legal experts can provide valuable insights into the intricacies of these frameworks, ensuring that all labeling practices are in accordance with current standards. As Crystal Maira, a Senior Consultant, asserts, "As deadlines for adherence are quickly approaching and add a significant workload to cosmetic producers, we recommend you begin preparing now for the requirements."
Actionable Tip: Develop a regulatory checklist based on the legal requirements pertinent to your target markets. This checklist should encompass elements such as annual item listing renewals post-December 29, 2023, and should be routinely updated to reflect any regulatory changes.
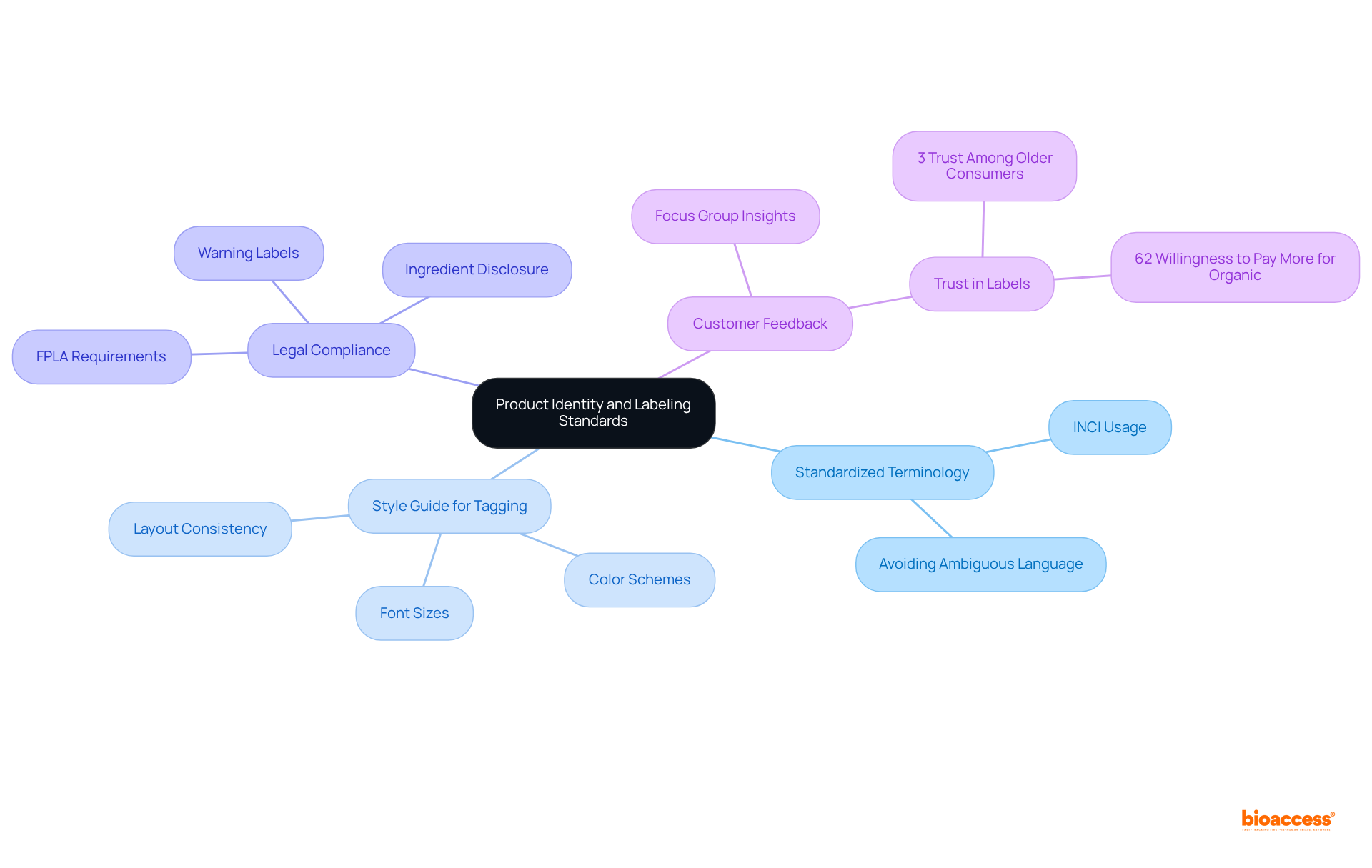
Creating distinct item identity and guidelines for labeling translation is crucial for adherence and customer trust. The process of labeling translation must accurately represent the item's purpose, benefits, and usage instructions. This necessitates the use of standardized terminology, such as the International Nomenclature of Cosmetic Ingredients (INCI), for labeling translation while avoiding ambiguous language that could mislead consumers. Additionally, implementing a style guide for tagging that outlines font sizes, colors, and layout is essential to ensure consistency across all products. It is important to note that the Fair Packaging and Labeling Act (FPLA) mandates specific labeling translation requirements that must be adhered to for legal compliance.
Actionable Tip: Organizing focus groups with customers can offer valuable insights into label designs and language. Given that only 3% of older individuals completely trust voluntary labels, this feedback can help identify areas of confusion and enhance overall clarity, ensuring that labels align with expectations. Furthermore, with 62% of younger buyers prepared to spend extra for certified personal care items containing organic ingredients, transparent marking standards can significantly improve trust and boost sales.
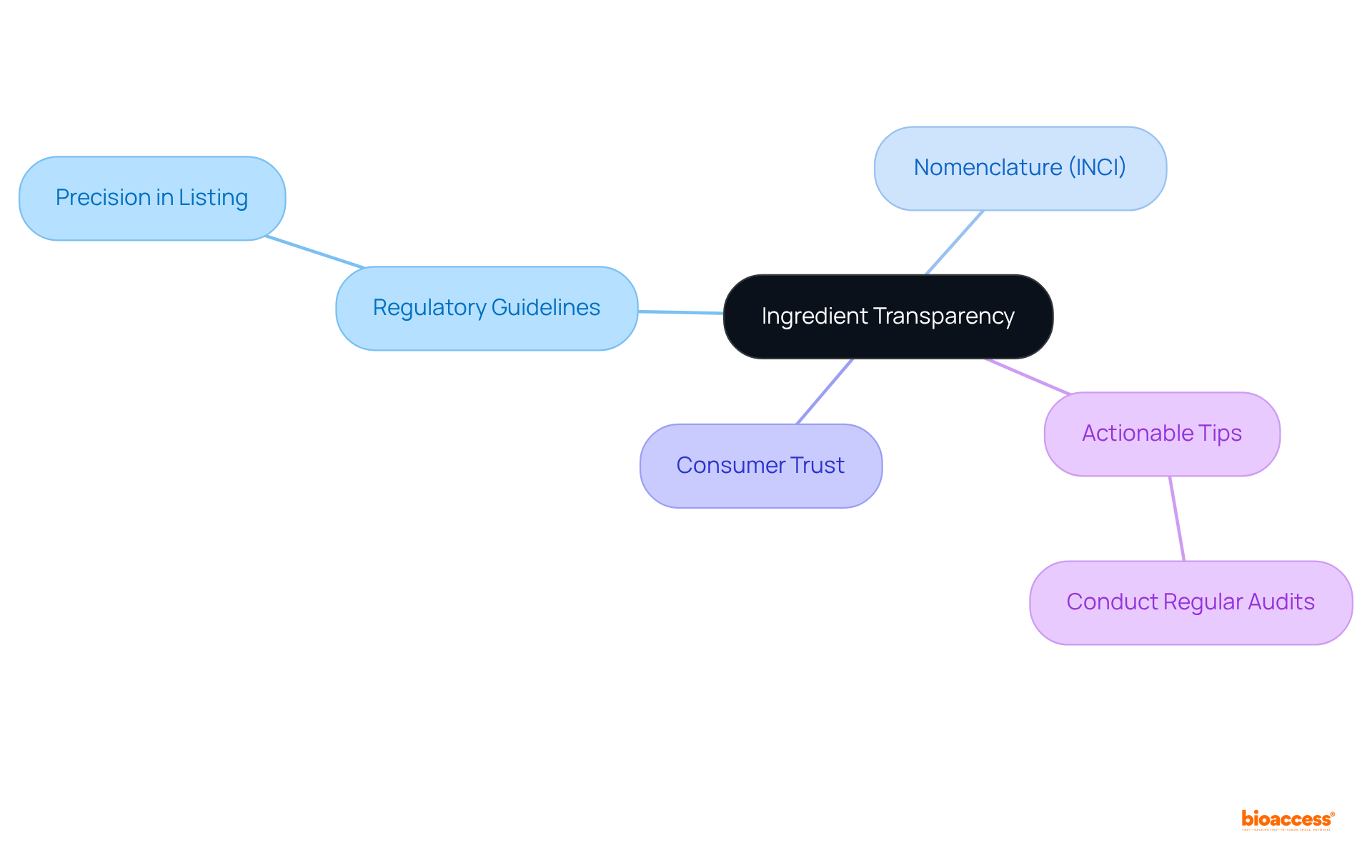
Ingredient transparency is a pivotal element of effective labeling translation. It is imperative that all ingredients are listed with precision and in strict accordance with regulatory guidelines for labeling translation. This entails the use of appropriate nomenclature, such as INCI (International Nomenclature of Cosmetic Ingredients) names, to eliminate any potential confusion. Furthermore, providing information regarding the source and purpose of each ingredient can significantly enhance consumer trust and understanding.
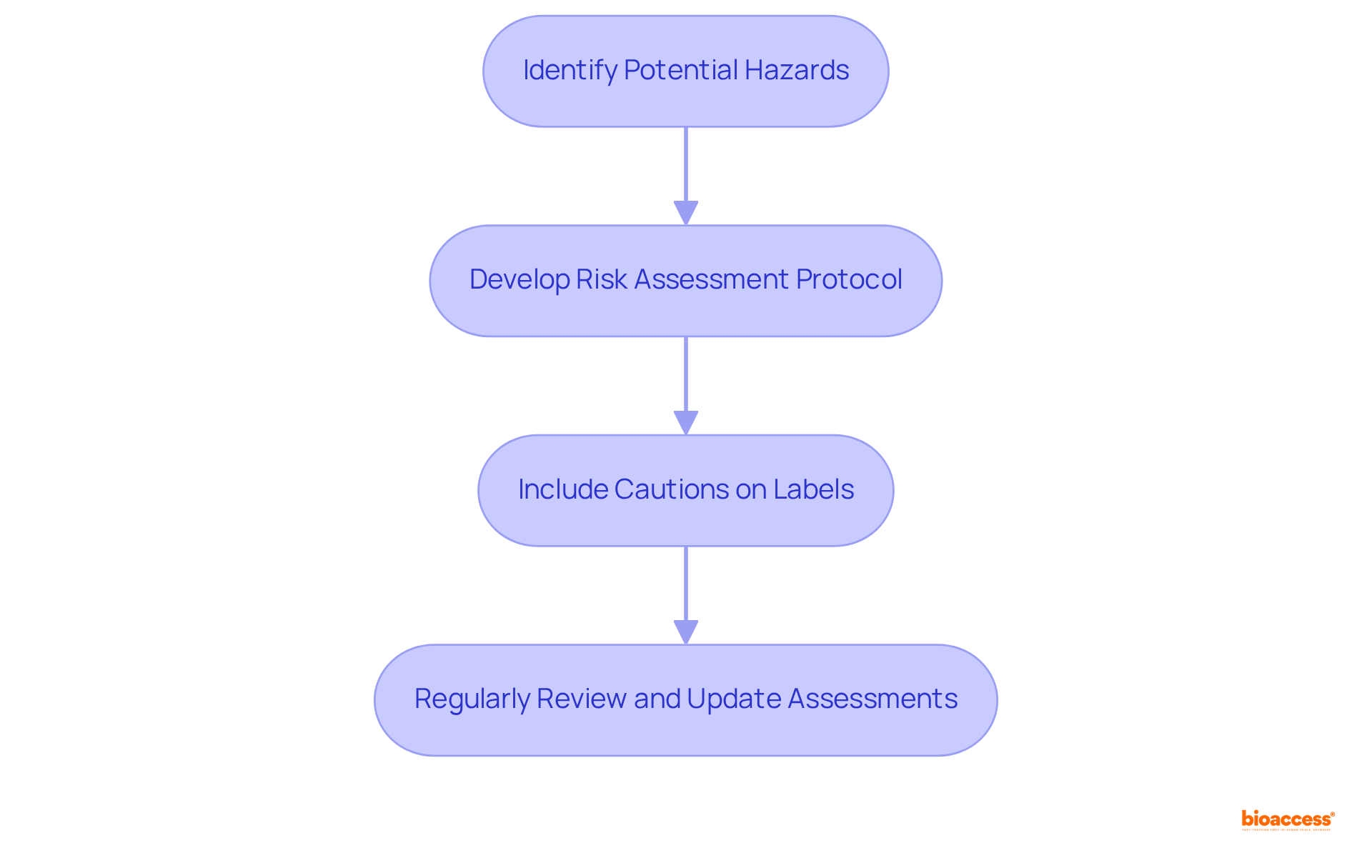
It is essential to address any potential risks related to cosmetic items on the labels. This includes providing necessary cautions, such as allergy warnings or usage instructions that mitigate risks. Clear communication of these hazards not only meets regulatory requirements but also safeguards individuals from potential harm. Statistics reveal that a substantial percentage of consumers remain unaware of the dangers linked to cosmetic items, underscoring the necessity for clear hazard communication. Labels must be designed to stand out, ensuring that cautionary statements are easily visible and understandable. As noted by experts, the Tobacco Industry (TI) strives to portray itself as socially responsible, which emphasizes the critical importance of transparency in labeling practices.
Actionable Tip:
Understanding and implementing effective labeling translation practices is crucial for cosmetic businesses aiming to maintain compliance and foster consumer trust. This article has underscored the significance of navigating regulatory frameworks, ensuring clear product identity, and providing ingredient transparency—elements that are essential for successful labeling in the cosmetic industry.
Key insights discussed include:
Ultimately, cosmetic businesses must prioritize compliance and clarity in their labeling practices. By developing comprehensive checklists, organizing customer feedback sessions, and conducting regular audits, companies can ensure their labels meet regulatory standards while resonating with consumers. A commitment to transparency and accuracy in labeling will not only protect consumers but also foster brand loyalty in an increasingly competitive market.
What are the key regulatory frameworks for cosmetic labeling?
The key regulatory frameworks include the FDA rules in the United States and the EU Cosmetics Regulation in Europe, which outline criteria for product identification, permissible claims, ingredient listings, and safety evaluations.
What is the deadline for businesses to register with the FDA?
All businesses involved in the production or handling of cosmetic products must register with the FDA by December 29, 2023.
Why is it important for companies to stay informed about cosmetic regulations?
It is important because regulations are subject to change, and staying informed helps avoid costly errors and ensures compliance with current standards.
How can companies ensure their labeling practices comply with regulations?
Companies can engage with regulatory consultants or legal experts to gain insights into the intricacies of the regulatory frameworks and ensure compliance with labeling practices.
What is a recommended action for companies regarding regulatory compliance?
It is advisable to develop a regulatory checklist based on legal requirements for target markets, which should include elements like annual item listing renewals and be routinely updated to reflect any regulatory changes.