


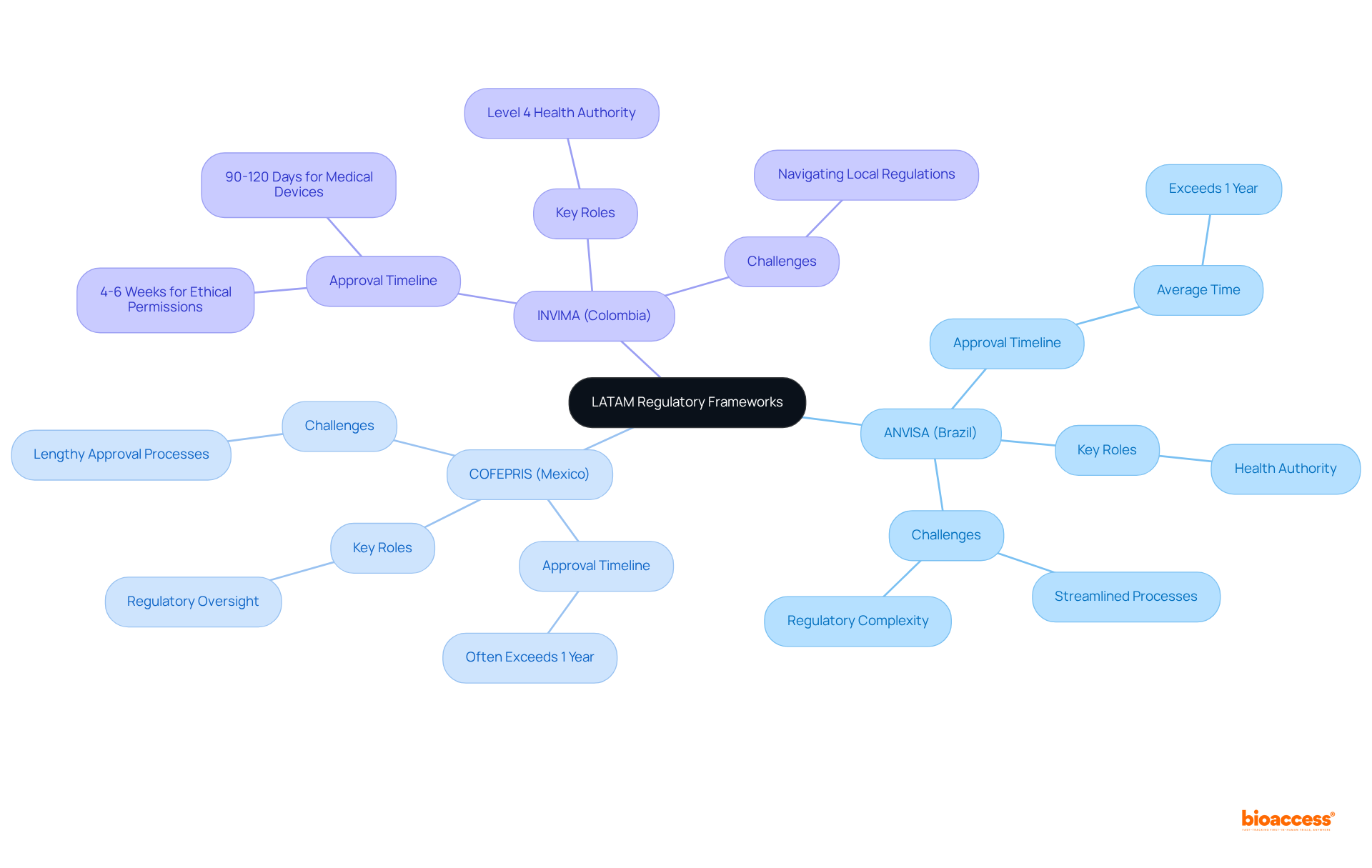
The article underscores the complexities inherent in LATAM's regulatory frameworks for Medtech innovations, with a focus on the distinct requirements and timelines mandated by governing bodies such as:
Understanding these local regulations is crucial for expediting market entry and enhancing competitiveness. Successful partnerships exemplified in the region have significantly reduced patient recruitment times and improved access to innovative treatments, illustrating the critical role of collaboration in navigating these challenges.
The regulatory landscape in Latin America (LATAM) presents a complex web of varying frameworks, each governed by its own set of rules and timelines for medical device approvals. For Medtech innovators, grasping these nuances is not merely beneficial; it is essential, as the differences can significantly influence market entry and product launch strategies.
Yet, a pertinent question arises: how can companies effectively navigate this intricate environment to harness the region's potential for expedited approvals and diverse patient populations? This article explores the LATAM regulatory strategist comparison chart, delving into the opportunities and challenges that lie ahead for those aiming to thrive in this dynamic market.
The governance structure in Latin America (LATAM) presents a complex tapestry of frameworks that differ markedly across nations. The principal governing bodies include:
Each enforces distinct requirements and timelines for medical device authorizations. For instance, INVIMA is recognized as a Level 4 health authority by PAHO/WHO and can provide ethical permissions in a mere 4-6 weeks. In contrast, COFEPRIS and ANVISA often have longer timelines, frequently exceeding a year for official endorsements. In Colombia, the average approval time for medical devices can extend to 90-120 days, while Brazil's processes, although more streamlined, still present notable challenges.
Effectively navigating these governing bodies is crucial for Medtech innovators, as illustrated in the latam regulatory strategist comparison chart. A prime example is the partnership between bioaccess® and GlobalCare Clinical Trials in Colombia, which achieved a remarkable 50% reduction in patient recruitment time. This underscores the advantages of local expertise in overcoming compliance challenges. Furthermore, the FIFARMA W.A.I.T. Indicator 2024 report reveals that while 61% of internationally endorsed medicines have local authorization in at least one Latin American nation, only 35% are accessible to the public, highlighting significant delays in obtaining innovative treatments.
Industry experts stress the necessity of understanding local regulations, as outlined in the latam regulatory strategist comparison chart, to facilitate smoother market entry. As one expert aptly stated, 'Understanding the nuances of local regulations is key to successful market entry.' This insight is vital as Medtech companies aim to leverage LATAM's regulatory speed and diverse patient populations for clinical trials and product launches, ultimately enhancing their competitiveness in the global market.
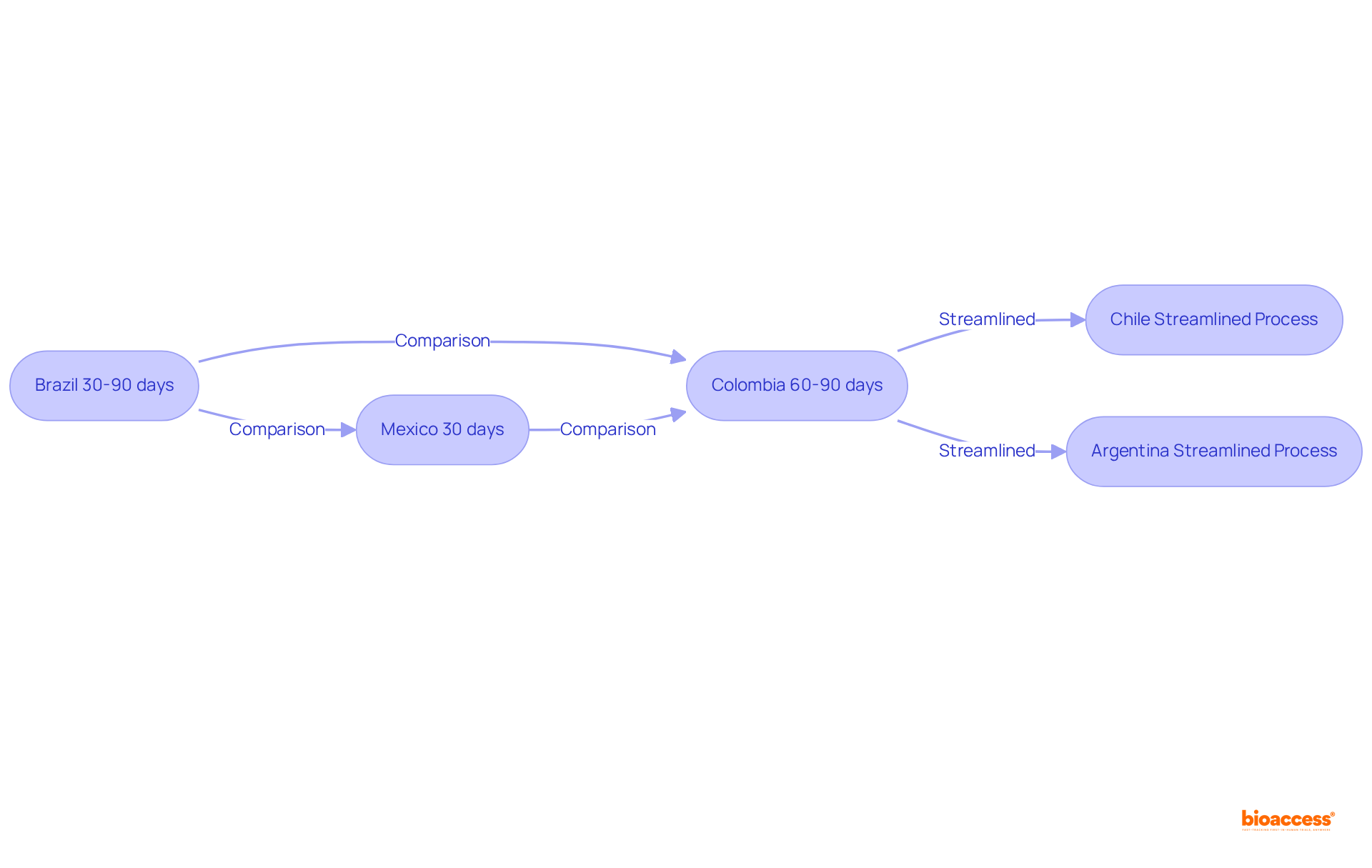
In Brazil, the authorization process for medical devices typically spans 30 to 90 days, contingent upon the device's classification. Conversely, Mexico's COFEPRIS is renowned for its expedited authorizations, often completing the process in as little as 30 days for innovative products. In Colombia, INVIMA generally requires 60 to 90 days for similar approvals, whereas ethical approvals managed by bioaccess® are delivered in a mere 4-6 weeks.
These variations underscore the critical necessity for Medtech firms to strategically select countries for clinical trials, guided by the latam regulatory strategist comparison chart, based on their urgency for market entry. Moreover, Colombia's significant population and universal healthcare system facilitate effective subject recruitment for clinical trials, rendering it a compelling option for Medtech firms.
Additionally, countries like Chile and Argentina are progressively adopting more streamlined processes, thereby enhancing their attractiveness for conducting early feasibility studies. With over 20 years of experience in Medtech, bioaccess® leads the way in comprehensive clinical trial management services, encompassing:
This empowers Medtech firms to adeptly navigate the complexities of the Latin American compliance landscape with confidence.
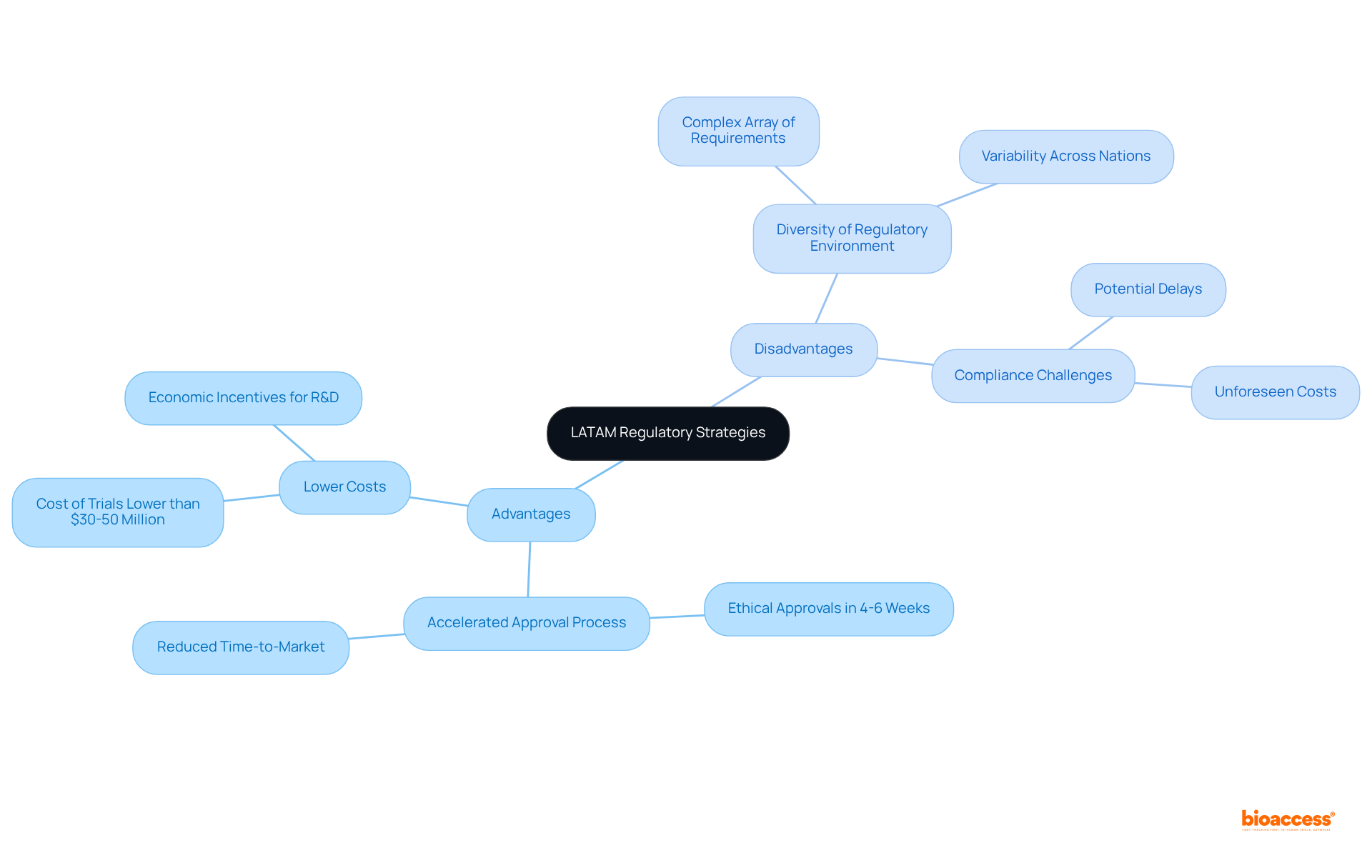
One of the primary advantages of pursuing compliance strategies in Latin America lies in the accelerated approval process, where ethical approvals can be secured in as little as 4 to 6 weeks. This swift timeline significantly reduces the time-to-market for innovative medical technologies, presenting an appealing opportunity for businesses.
However, the diversity of the regulatory environment in Latin America, highlighted in the latam regulatory strategist comparison chart, necessitates that companies navigate a complex array of requirements and standards across various nations.
While the overall cost of conducting clinical trials in Latin America is typically lower than in the U.S. and Europe, unforeseen compliance challenges can lead to delays and increased expenses. For instance, while the average cost of clinical trials in Latin America may be significantly less than the usual $30 to $50 million range observed in the U.S., firms must remain vigilant regarding potential compliance hurdles that could impact their budgets.
A thorough understanding of each country's governance landscape is essential for optimizing strategies and minimizing risks, thereby enabling companies to effectively harness the benefits of conducting clinical trials in this region.
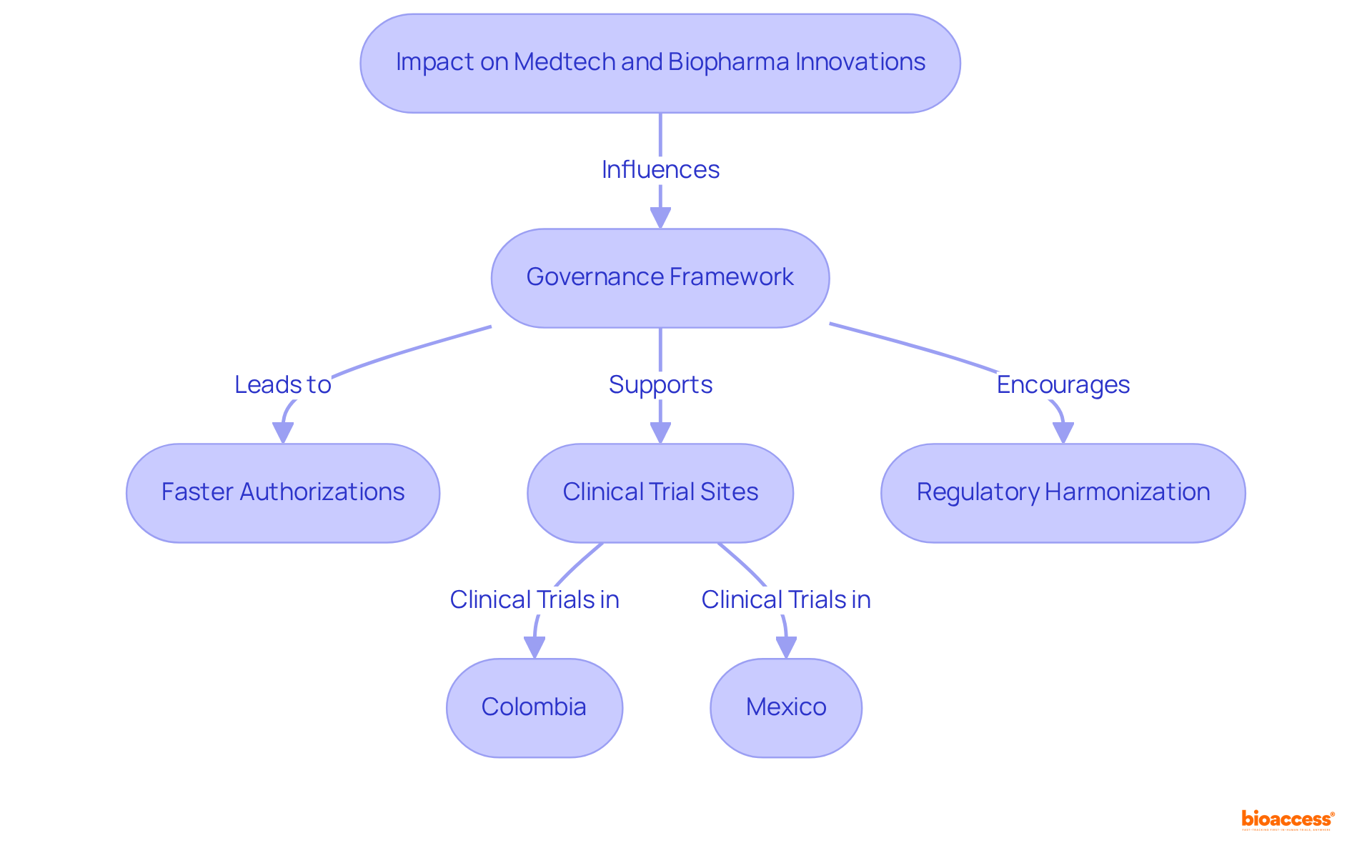
The governance framework in LATAM significantly influences advancements in Medtech and Biopharma, creating a unique environment that promotes faster authorizations and access to diverse patient populations. Countries such as Colombia and Mexico are emerging as leading sites for clinical trials, bolstered by their robust healthcare infrastructures and regulatory efficiencies. For instance, bioaccess® secures ethical consent in just 4-6 weeks, facilitating enrollment that is 50% quicker than in traditional markets. This expedited approval process not only accelerates the testing and enhancement of products in real-world settings but also fosters innovation by enabling companies to bring their solutions to market more swiftly.
Nevertheless, the varying compliance standards across Latin America can pose challenges for companies struggling to adapt quickly. The recent trend towards regulatory harmonization is a promising development, enhancing collaboration and potentially speeding up the introduction of new technologies. Case studies that showcase Chile's advancements in healthcare infrastructure and Mexico's expanding capabilities in clinical research highlight the region's potential to support innovative clinical trials. Furthermore, bioaccess® offers comprehensive services, including feasibility studies, project management, and compliance reviews, which are crucial for navigating these regulatory frameworks. Ultimately, the ability to adeptly maneuver through these oversight structures will be vital for the success of Medtech and Biopharma innovations in LATAM, as companies capitalize on the region's unique advantages to propel their advancements.
Navigating the regulatory landscape in Latin America is essential for Medtech innovators aiming to bring their products to market efficiently. The diverse frameworks established by governing bodies such as ANVISA in Brazil, COFEPRIS in Mexico, and INVIMA in Colombia create unique challenges and opportunities. Understanding these variations is crucial for companies seeking to leverage the region's potential for expedited approvals and access to diverse patient populations.
Key insights reveal that:
The importance of local expertise cannot be overstated, as demonstrated by successful partnerships that have significantly reduced patient recruitment times. Moreover, the ongoing trend toward regulatory harmonization signifies a promising shift that could enhance collaboration and streamline processes across the region.
The implications of these regulatory frameworks extend beyond mere compliance; they have the power to shape the future of Medtech and Biopharma innovations in LATAM. As companies adapt to these complexities and capitalize on the advantages offered by the region, the potential for accelerated product development and enhanced patient access becomes increasingly attainable. Embracing the challenges of LATAM’s regulatory environment not only fosters innovation but also positions businesses to thrive in a competitive global market.
What are the main governing bodies for medical device regulations in Latin America (LATAM)?
The principal governing bodies include ANVISA in Brazil, COFEPRIS in Mexico, and INVIMA in Colombia.
How do the timelines for medical device authorizations vary among LATAM countries?
INVIMA can provide ethical permissions in 4-6 weeks, while COFEPRIS and ANVISA often have longer timelines, frequently exceeding a year. In Colombia, the average approval time for medical devices is 90-120 days, and Brazil's processes, although more streamlined, still present notable challenges.
Why is it important for Medtech innovators to navigate LATAM's regulatory frameworks?
Effectively navigating these governing bodies is crucial for Medtech innovators to overcome compliance challenges and facilitate smoother market entry.
Can you provide an example of a successful partnership in navigating LATAM regulations?
A prime example is the partnership between bioaccess® and GlobalCare Clinical Trials in Colombia, which achieved a 50% reduction in patient recruitment time, highlighting the advantages of local expertise.
What does the FIFARMA W.A.I.T. Indicator 2024 report reveal about medicine authorization in LATAM?
The report reveals that while 61% of internationally endorsed medicines have local authorization in at least one Latin American nation, only 35% are accessible to the public, indicating significant delays in obtaining innovative treatments.
What do industry experts emphasize regarding local regulations in LATAM?
Industry experts stress the necessity of understanding local regulations to facilitate smoother market entry, as understanding the nuances is key to successful market entry for Medtech companies.
How can understanding LATAM's regulatory speed benefit Medtech companies?
By leveraging LATAM's regulatory speed and diverse patient populations for clinical trials and product launches, Medtech companies can enhance their competitiveness in the global market.