


Navigating the complex landscape of medical device labeling is crucial for manufacturers who want to ensure compliance and protect patient safety. The regulations outlined in 21 CFR 809 establish essential requirements that not only safeguard consumers but also bolster the credibility of medical products in a competitive market.
With guidelines constantly evolving and the risk of costly compliance failures looming, how can manufacturers effectively master these regulations and sidestep common pitfalls?
This article explores the intricacies of 21 CFR 809, providing a comprehensive guide to crafting compliant medical device labels that meet regulatory standards while minimizing risks.
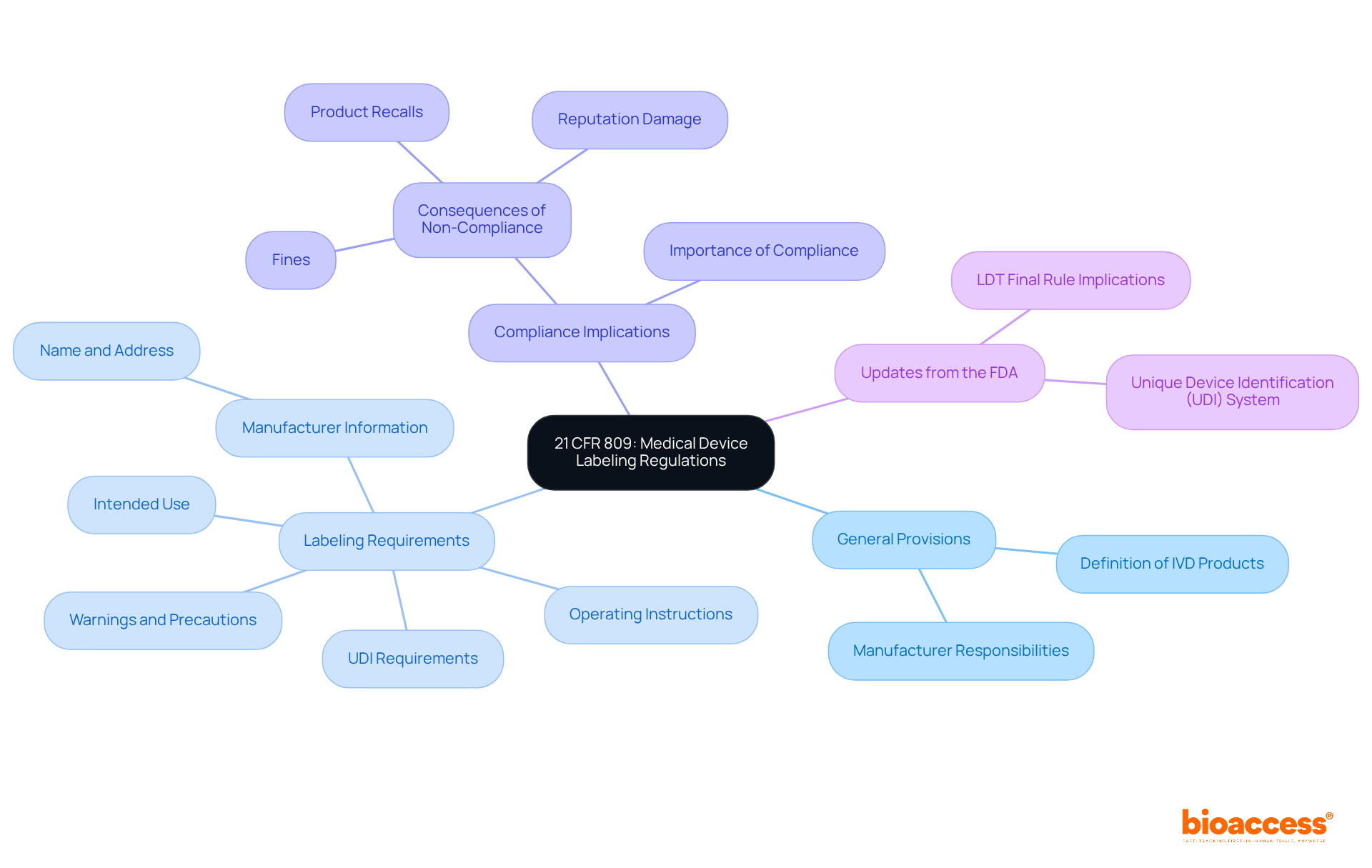
The labeling requirements for in vitro diagnostic (IVD) products are outlined in 21 CFR 809, which establishes essential information that must be present on labels. This includes the device's intended use, instructions for use, and necessary warnings. Understanding these regulations is crucial for ensuring compliance and mitigating legal risks. Key components of 21 CFR 809 compliance include:
Recent updates from the FDA highlight the ongoing evolution of marking requirements, particularly regarding the Unique Device Identification (UDI) system. This mandates that all medical devices, including IVDs, display a UDI on their tags, aiming to enhance traceability and improve patient safety.
Effective identification strategies often involve a comprehensive understanding of the regulatory landscape and proactive measures to ensure compliance with 21 CFR 809. For instance, organizations that implement robust Quality Management Systems (QMS) can streamline their documentation processes, ensuring that all essential information is accurately represented and easily accessible.
In summary, mastering the intricacies of 21 CFR 809 is essential for medical product manufacturers. By prioritizing compliance and staying informed about regulatory changes, companies can effectively navigate the complexities of medical equipment labeling and enhance their market presence.
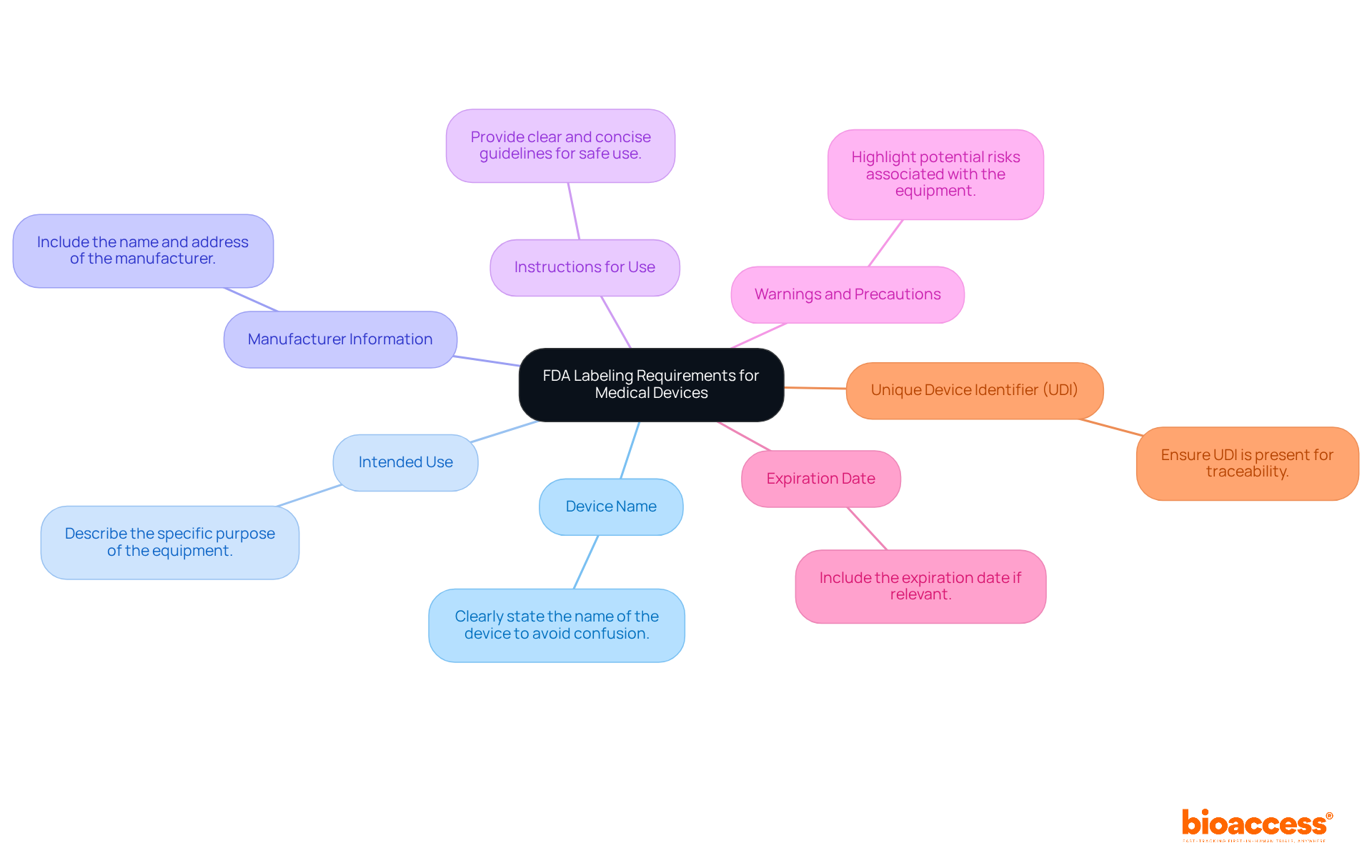
To create compliant medical device labels, manufacturers must include several key elements that align with FDA regulations:
These identification components are not only regulatory necessities but also crucial for ensuring that users possess all the essential information to operate the apparatus safely and effectively. Adherence to UDI regulations is especially crucial, as research shows that labeling mistakes rank among the top five most frequent citations given to medical equipment companies. By following these guidelines, manufacturers can enhance traceability, improve market confidence, and contribute to safer patient outcomes globally.
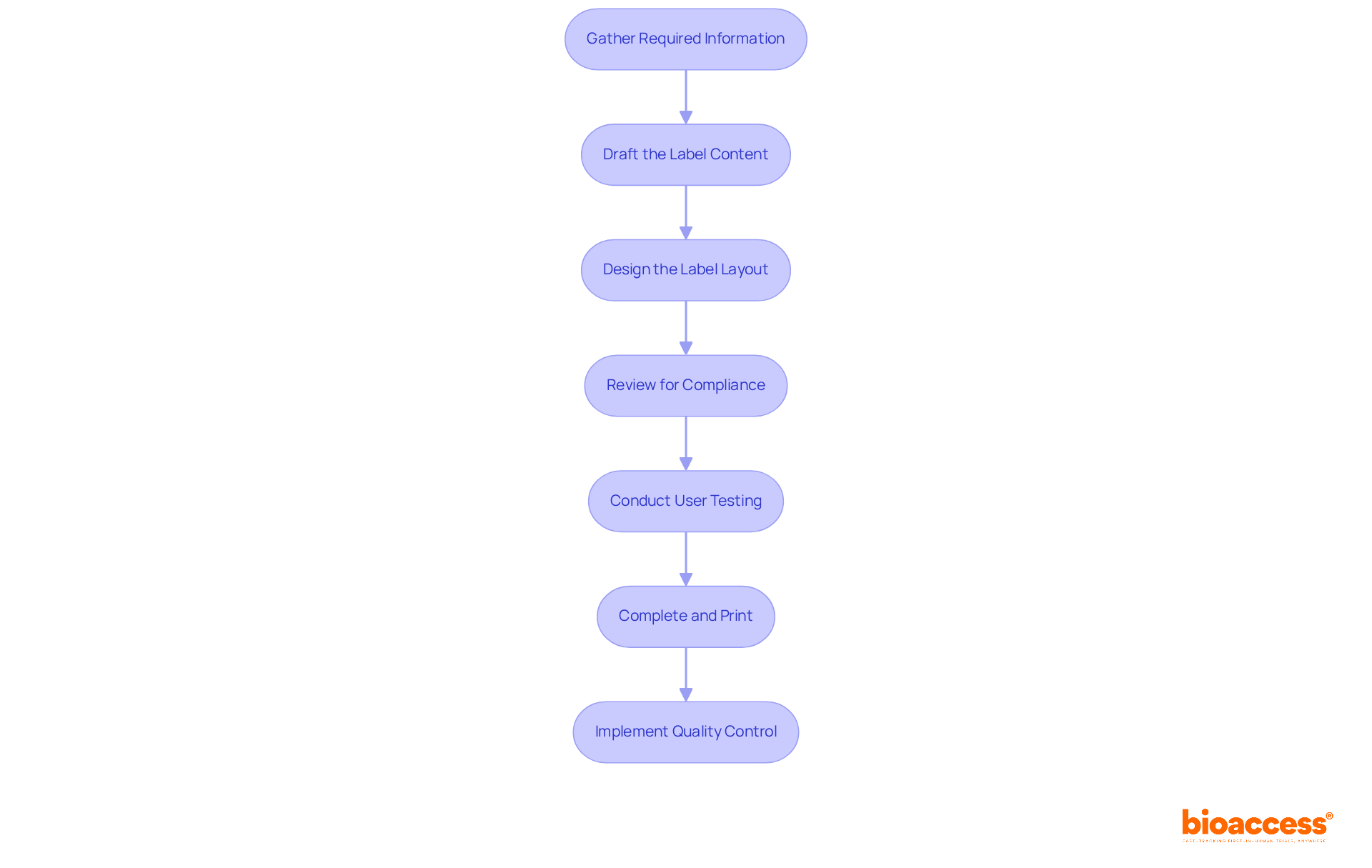
Creating compliant medical device labels is crucial for ensuring safety and effectiveness in clinical research. Follow these essential steps to guarantee your labels meet regulatory standards and effectively communicate vital information:
Gather Required Information: Start by collecting all necessary details about the device, including its name, intended use, warnings or precautions, manufacturer information, and lot or serial number. This foundational step ensures that all critical elements are addressed.
Draft the Label Content: Write clear and concise text for each required element, ensuring it is easy to understand. Effective labeling can significantly reduce the risk of medication errors, which are often linked to mislabeling.
Design the Label Layout: Create a visually appealing layout that highlights critical information while adhering to FDA guidelines regarding font size and contrast. A well-crafted tag improves usability and adherence.
Review for Compliance: Cross-check the information against FDA requirements to ensure all necessary details are included. This includes adhering to the standards set forth in 21 CFR 809, along with Parts 801 and 820, which outline essential labeling requirements.
Conduct User Testing: If feasible, test the tag with potential users to gather feedback on clarity and usability. Interacting with users can offer valuable insights that enhance the effectiveness of tags. As Andrew Wootten, a Quality and Regulatory Functions Expert, emphasizes, "User feedback is essential for ensuring that markings communicate effectively and safely."
Complete and Print: Once all modifications are made, finalize the design and print the tags using durable materials that endure wear and tear. This step is crucial for maintaining identification integrity throughout the product's lifecycle.
Implement Quality Control: Establish a quality assurance process to regularly review tags for compliance and accuracy. Continuous monitoring helps prevent labeling errors, which account for approximately 8% of all FDA recalls as of 2025. Regular reviews guarantee that tags stay in accordance with changing regulations and standards.
By adhering to these steps, manufacturers can ensure their medical product tags are not only compliant but also efficient in communicating vital information to users.
Common labeling issues and compliance challenges in medical device labeling include:
Troubleshooting Steps:
As Bill Harrison, CEO of ComplianceBridge, highlights, "You need to assess adherence not as a cost, but as a means of saving money." This viewpoint is essential in comprehending the significance of tackling identification challenges. Additionally, a case study involving Lionbridge's work with a global biopharmaceutical company illustrates how streamlined processes can mitigate compliance challenges in clinical labeling, ultimately enhancing product safety and user trust.

Mastering the requirements of 21 CFR 809 is crucial for manufacturers of medical devices, especially in vitro diagnostics. This framework not only outlines essential labeling information but also acts as a safeguard for user safety and regulatory compliance. By understanding and implementing these guidelines, companies can reduce legal risks and bolster their credibility in the market.
The article explores the critical components of medical device labeling, stressing the necessity of including:
The Unique Device Identifier (UDI) is also highlighted as a vital element for traceability and compliance. Following a step-by-step process for label creation ensures that all essential information is accurately represented, ultimately contributing to safer patient outcomes and enhanced market confidence.
In a landscape where regulatory requirements are continually evolving, proactive engagement with compliance measures is essential. Manufacturers should regularly review and update their labeling practices, engage stakeholders, and implement feedback mechanisms to refine their processes. By prioritizing compliance and user understanding, the medical device industry can cultivate a culture of safety and reliability, reinforcing the importance of adhering to 21 CFR 809 regulations not merely as a legal obligation but as a commitment to public health.
What does 21 CFR 809 regulate?
21 CFR 809 regulates the labeling requirements for in vitro diagnostic (IVD) products, outlining essential information that must be present on labels, including intended use, instructions for use, and necessary warnings.
What are the key components of compliance with 21 CFR 809?
Key components include understanding general provisions that define IVD products, adhering to labeling requirements such as including the manufacturer's name, intended use, operating instructions, and warnings, as well as recognizing the compliance implications of non-compliance.
What are the consequences of non-compliance with 21 CFR 809?
Non-compliance can result in significant consequences such as fines, product recalls, and damage to a company's reputation, as well as loss of market access and consumer trust.
What recent updates have been made regarding medical device labeling?
Recent updates from the FDA include the implementation of the Unique Device Identification (UDI) system, which requires all medical devices, including IVDs, to display a UDI on their labels to enhance traceability and improve patient safety.
How can organizations ensure compliance with 21 CFR 809?
Organizations can ensure compliance by implementing robust Quality Management Systems (QMS) that streamline documentation processes, ensuring that all essential information is accurately represented and easily accessible.
Why is understanding 21 CFR 809 important for medical product manufacturers?
Understanding 21 CFR 809 is crucial for manufacturers to effectively navigate the complexities of medical equipment labeling, prioritize compliance, stay informed about regulatory changes, and enhance their market presence.