


This article highlights the critical importance of mastering 21 CFR 814, which delineates the premarket approval (PMA) process for medical devices. Understanding and adhering to its rigorous requirements is essential for clinical researchers aiming to ensure the safety and effectiveness of these devices. This includes conducting comprehensive clinical trials and maintaining clear communication with the FDA.
In the ever-evolving Medtech landscape, navigating the approval process can be daunting. However, by grasping these regulations, researchers can significantly enhance patient outcomes. The ability to effectively manage the PMA process not only fosters trust but also positions researchers as leaders in clinical innovation.
Ultimately, collaboration and proactive engagement with regulatory bodies are paramount. As you consider your own challenges in clinical research, reflect on how mastering these guidelines can pave the way for success. Embrace the opportunity to elevate your practice and contribute to the advancement of medical technology.
Navigating the complex landscape of medical device approval presents a significant challenge for clinical researchers, especially when it comes to grasping the critical role of 21 CFR 814. This regulation delineates the rigorous premarket approval (PMA) process, ensuring that high-risk devices adhere to stringent safety and effectiveness standards. As the FDA continues to refine its guidelines, the stakes are considerable—noncompliance can result in substantial delays or even outright rejection of applications.
So, how can researchers effectively navigate these complexities to boost their chances of successful device approval? By mastering the intricacies of the approval process, they can not only enhance their prospects but also contribute meaningfully to advancements in healthcare. Understanding these regulations is not just beneficial; it’s essential for fostering innovation and ensuring patient safety.
The premarket approval (PMA) procedure for medical devices intended for human use is outlined in 21 CFR 814, which plays a crucial role in ensuring the safety and effectiveness of high-risk Class III devices. This regulation is essential for clinical researchers as it delineates the rigorous requirements necessary for FDA submissions. Key elements include:
All of which uphold the integrity of the medical device approval system.
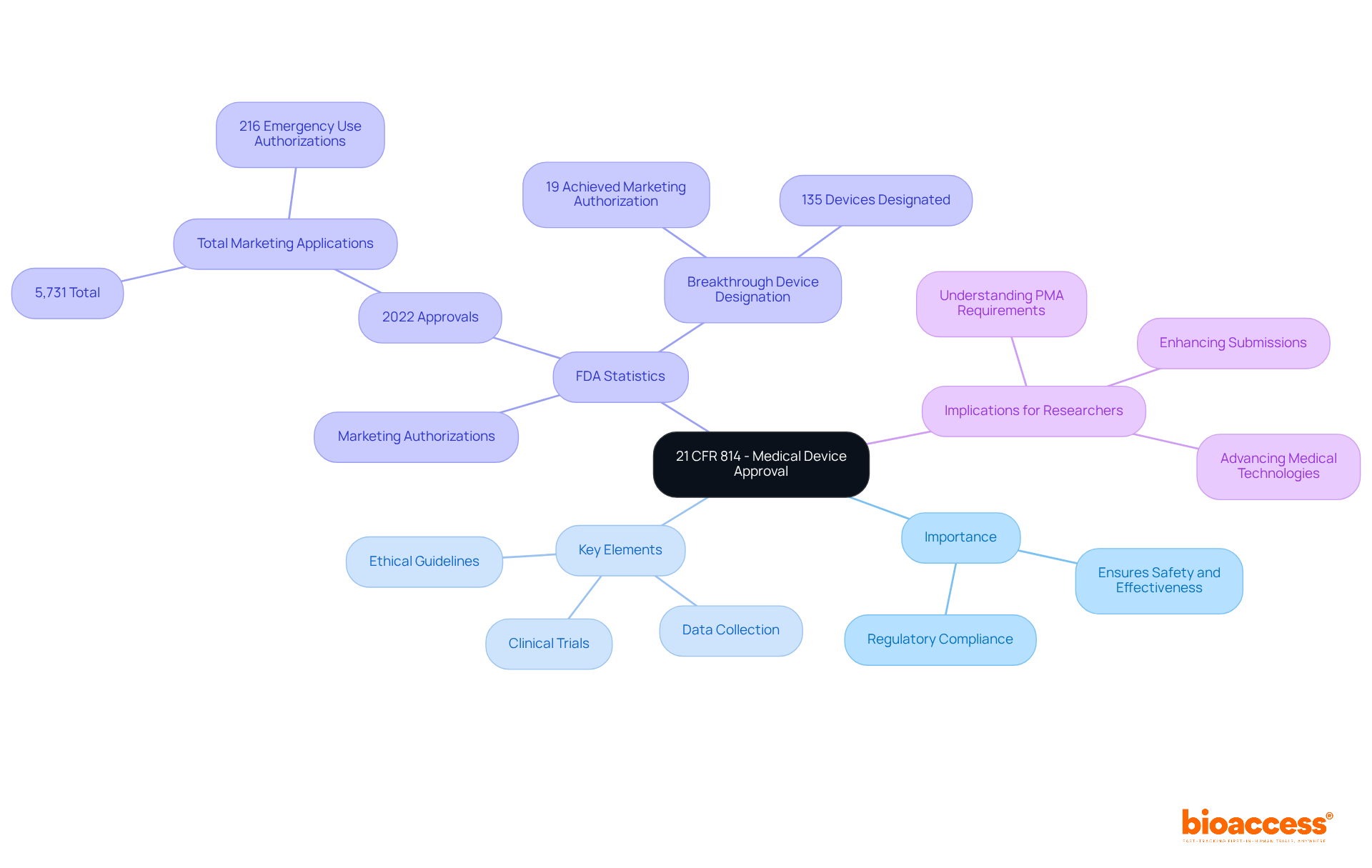
Recent updates to the approval process in 21 CFR 814 reflect the FDA's commitment to streamlining while maintaining high safety standards. In 2022, the FDA approved a total of 5,731 marketing applications, including 216 Emergency Use Authorizations related to COVID-19, showcasing the agency's responsiveness to public health needs. Notably, the average time for a PMA review is set at 180 days, although major amendments can significantly extend this period.
The effectiveness of the regulation is demonstrated by successful medical device approvals under 21 CFR 814. For instance, in 2022:
This indicates a successful pathway for innovative medical technologies. Additionally, the FDA's focus on quality scientific writing and accurate clinical information is essential, as almost 35 percent of PMA applications encountered difficulties due to insufficient data or ambiguity.
For clinical researchers aiming to navigate the complexities of device approval effectively, understanding 21 CFR 814 is vital. By adhering to these regulations, researchers can enhance their submissions, ultimately contributing to the advancement of medical technologies that improve patient outcomes.
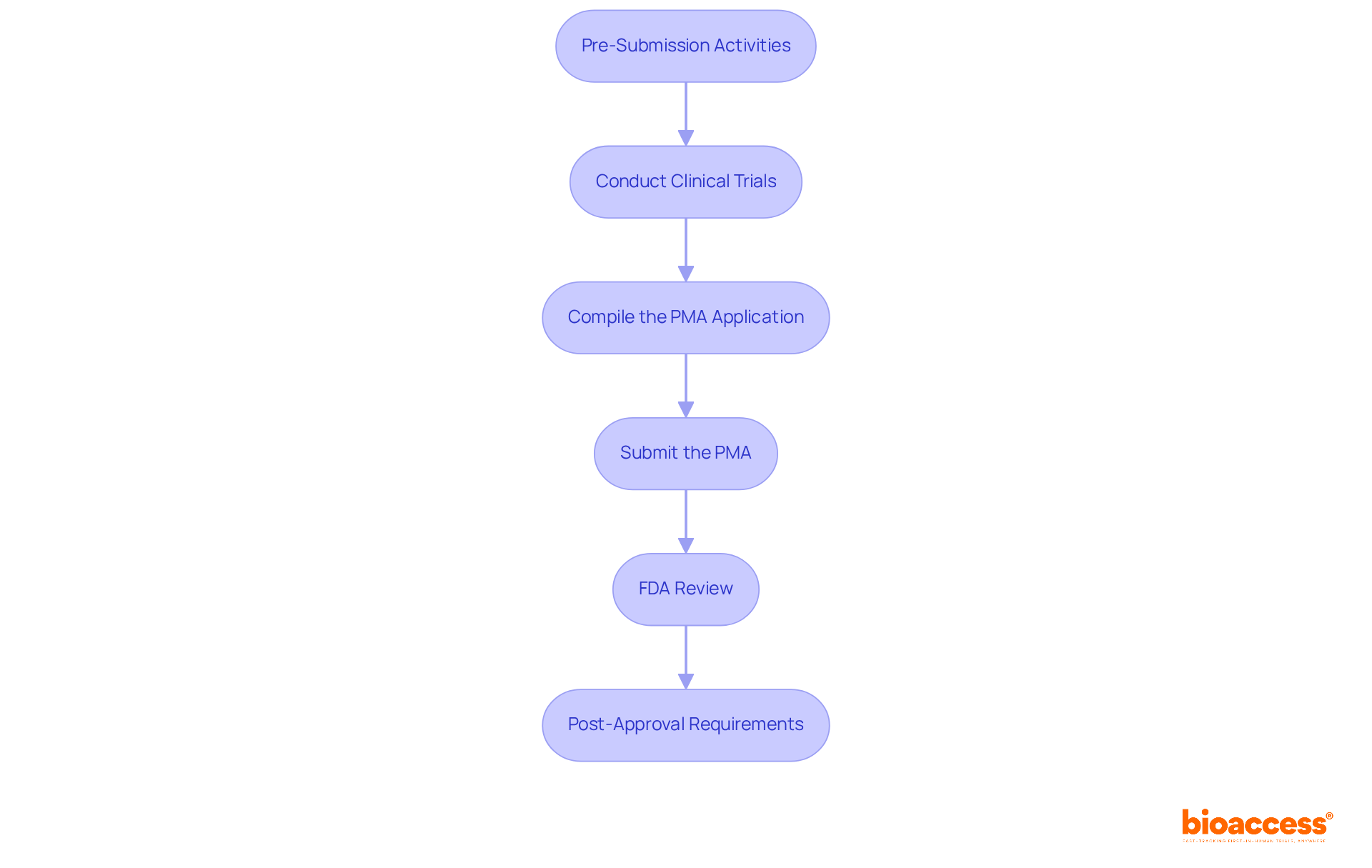
The Premarket Approval (PMA) process is vital for ensuring the safety and effectiveness of medical devices. It involves several critical steps that every Medtech company must navigate:
Pre-Submission Activities: Start by engaging early with the FDA to discuss your device and gather valuable feedback on your proposed study design. This proactive communication clarifies regulatory expectations and streamlines the approval process. Understanding local regulatory nuances in Latin America is crucial, as each country may have different requirements that can impact your strategy.
Conduct Clinical Trials: Implement well-structured clinical trials that adhere to Good Clinical Practice (GCP) guidelines. Collecting robust data that thoroughly addresses the safety and effectiveness of the device is essential, as this will form the backbone of your PMA application. Notably, bioaccess® is leading the way in facilitating medical device clinical trials in Latin America, achieving patient enrollment 50% faster than Western sites and saving $25K per patient with FDA-ready data.
Compile the PMA Application: Assemble a comprehensive PMA application that includes detailed clinical data, manufacturing information, and proposed labeling. Ensure that all sections are meticulously completed and conform to FDA formatting requirements to avoid delays. Keep in mind that 30-35% of entries fail the initial acceptance review, making thorough preparation crucial.
Submit the PMA: Electronically send your application through the FDA’s portal, ensuring that all required documentation is included and the necessary fees are paid.
FDA Review: The FDA will conduct a thorough review of your application, which includes three critical steps: acceptance review, substantive review, and decision-making. Be prepared to respond quickly to any questions or requests for further details to ensure a seamless review. The average turnaround time for PMA submissions is approximately 363.2 days. In Latin America, utilizing local knowledge can assist in navigating the review procedures more effectively.
Post-Approval Requirements: Once approved, adhere to any post-approval studies or reporting obligations mandated by the FDA to maintain compliance and ensure ongoing safety and effectiveness monitoring of your device. Risk management must be integrated throughout the regulatory framework as per ISO 14971 requirements, and ongoing vigilance is necessary to meet regulatory guidance. Engaging with local stakeholders can also enhance compliance and market access strategies in the diverse Latin American landscape.
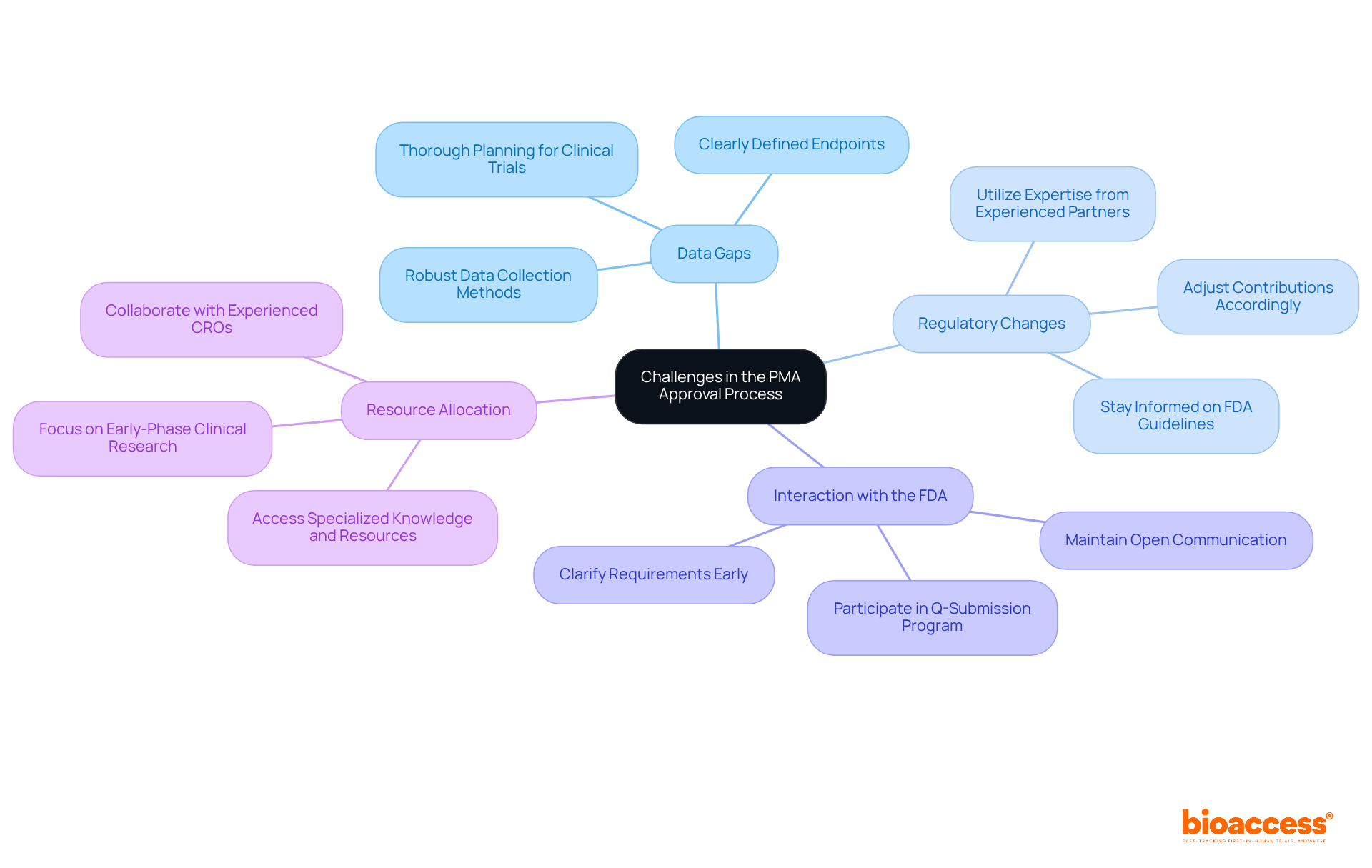
Navigating the PMA process presents several significant challenges:
Data Gaps: Incomplete or insufficient data often delays the approval process. To mitigate this risk, thorough planning and execution of clinical trials are essential. Clearly defined endpoints and robust data collection methods are crucial. Statistics show that insufficient clinical data is a primary challenge in PMA applications, necessitating comprehensive evidence for safety and effectiveness. Collaborating with experienced partners like bioaccess®, known for managing clinical trials—including the first-in-human study for Avantec Vascular—can enhance data integrity and completeness.
Regulatory Changes: The regulatory landscape is constantly evolving, with the FDA frequently updating guidelines. Staying informed about these changes is crucial for adjusting contributions accordingly. For instance, the PMA review timeline can vary from six months to over a year, and any shifts in regulatory expectations can impact the approval timeline. bioaccess®'s expertise in navigating these complexities ensures that applications align with the latest regulatory requirements.
Interaction with the FDA: Effective dialogue with FDA representatives is vital to prevent misunderstandings that can hinder the submission procedure. Keeping open channels of communication enables clarification on unclear requirements and nurtures a cooperative relationship, which can accelerate the review. Participating in the Q-Submission Program can also assist in clarifying requirements and simplifying the PMA review. bioaccess® emphasizes the importance of this communication, leveraging its extensive experience to facilitate smoother interactions with regulatory bodies.
Resource Allocation: Limited resources can hinder the approval procedure, making it challenging to meet the rigorous demands of the PMA pathway. Collaborating with seasoned contract research organizations (CROs) such as bioaccess® offers access to specialized knowledge and resources, ensuring a more streamlined application. With a focus on early-phase clinical research, including First-In-Human and Pivotal Studies, bioaccess® helps bridge the gap between innovative medical technologies and regulatory compliance, facilitating faster approvals.
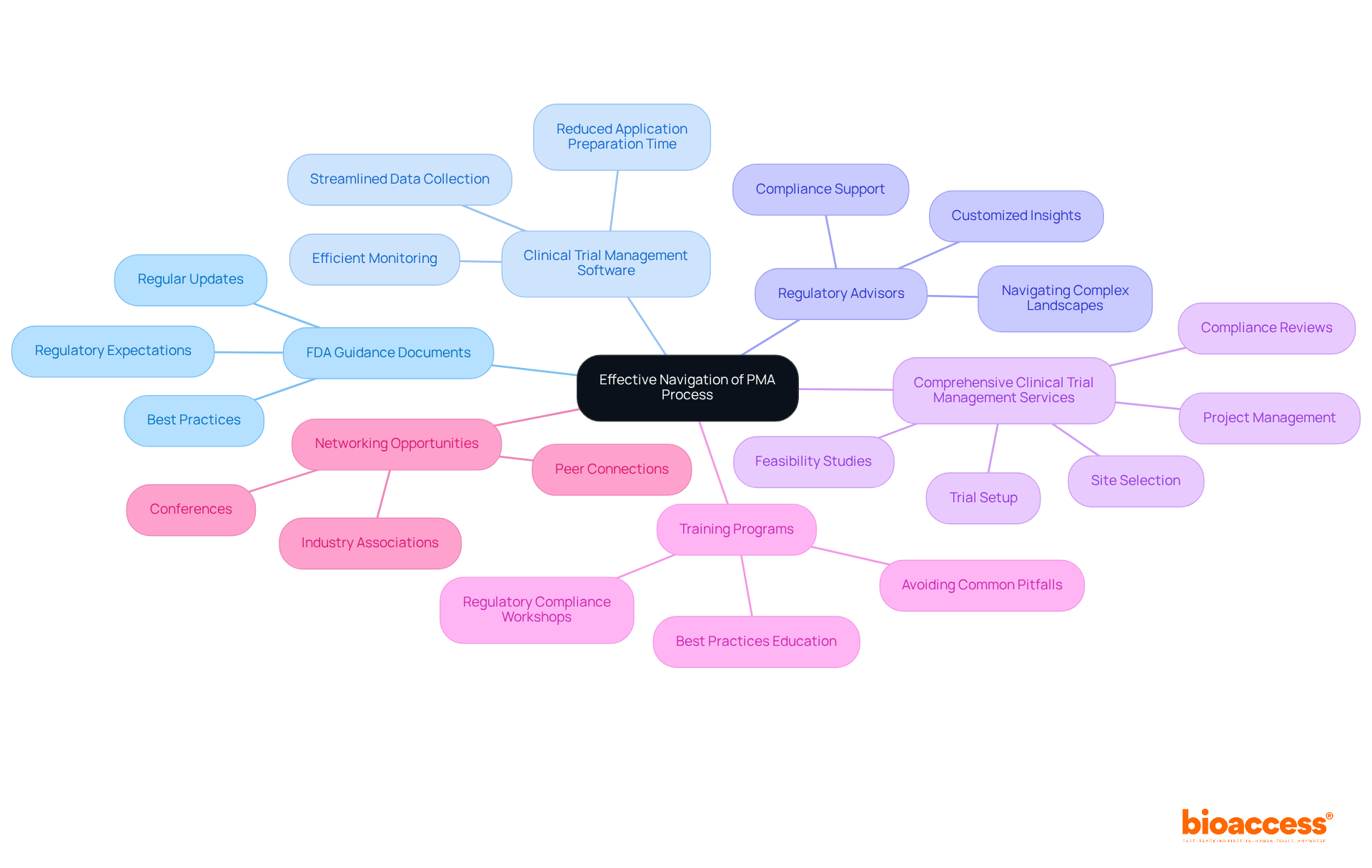
To effectively navigate the PMA process, it’s essential to leverage a range of tools and resources that can enhance your approach:
FDA Guidance Documents: Regularly reviewing the latest FDA guidance documents related to PMA applications is crucial. Staying updated on these documents outlines best practices and regulatory expectations that can significantly impact your approval timeline.
Clinical Trial Management Software: Implementing clinical trial management software can streamline data collection and monitor trial progress efficiently. Such solutions have been shown to reduce the time required for preparing applications, making them invaluable in the process.
Regulatory Advisors: Collaborating with regulatory advisors who specialize in PMA applications offers customized insights and support. Their expertise helps navigate complex regulatory landscapes and ensures compliance with all necessary requirements, ultimately enhancing the likelihood of successful submissions.
Comprehensive Clinical Trial Management Services: Leveraging services that encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting can significantly improve your capacity to meet regulatory requirements. This is particularly beneficial in regions like LATAM, the Balkans, and Australia, where navigating local regulations can be challenging.
Training Programs: Participating in training programs and workshops focused on regulatory compliance and clinical trial management can greatly enhance your team's capabilities. These programs often cover the latest regulatory changes and best practices, equipping your team with the knowledge needed to avoid common pitfalls in the PMA process.
Networking Opportunities: Joining industry associations and attending conferences can facilitate connections with peers and experts. These networking opportunities allow for the sharing of valuable experiences and insights, which can be instrumental in overcoming challenges associated with PMA submissions.
Understanding and mastering 21 CFR 814 is crucial for clinical researchers who want to navigate the intricate landscape of medical device approval. This regulation not only lays the groundwork for ensuring the safety and effectiveness of high-risk Class III devices but also underscores the necessity of rigorous clinical trials, meticulous data collection, and strict adherence to ethical guidelines. By grasping the nuances of 21 CFR 814, researchers can significantly enhance their submissions and contribute to the advancement of innovative medical technologies.
The article explores the step-by-step process of obtaining Premarket Approval (PMA), highlighting the critical stages from pre-submission activities to post-approval requirements. It addresses key challenges such as:
Offering practical solutions to overcome these hurdles. Utilizing tools and resources, including FDA guidance documents and clinical trial management software, can streamline the approval process and bolster the chances of success.
Ultimately, the significance of 21 CFR 814 goes beyond mere compliance; it embodies a commitment to patient safety and the advancement of healthcare. Clinical researchers are urged to engage with this regulation proactively, leveraging available resources and expertise to navigate the approval process effectively. By doing so, they not only facilitate the entry of innovative medical devices into the market but also play a vital role in enhancing patient outcomes and advancing public health.
What is 21 CFR 814 and why is it important?
21 CFR 814 outlines the premarket approval (PMA) procedure for medical devices intended for human use, ensuring the safety and effectiveness of high-risk Class III devices. It is crucial for clinical researchers as it specifies the rigorous requirements for FDA submissions.
What are the key requirements outlined in 21 CFR 814?
The key requirements include comprehensive clinical trials, meticulous data collection, and strict adherence to ethical guidelines, all of which uphold the integrity of the medical device approval system.
How has the FDA updated the approval process in 21 CFR 814?
Recent updates reflect the FDA's commitment to streamlining the approval process while maintaining high safety standards. In 2022, the FDA approved a total of 5,731 marketing applications, including 216 Emergency Use Authorizations related to COVID-19.
What is the average time for a PMA review under 21 CFR 814?
The average time for a PMA review is set at 180 days, although major amendments can significantly extend this review period.
How many devices received Breakthrough Device Designation in 2022?
In 2022, 135 devices received Breakthrough Device Designation, indicating a successful pathway for innovative medical technologies.
What percentage of PMA applications faced difficulties, and what were the common issues?
Almost 35 percent of PMA applications encountered difficulties due to insufficient data or ambiguity, highlighting the importance of quality scientific writing and accurate clinical information.
Why is understanding 21 CFR 814 vital for clinical researchers?
Understanding 21 CFR 814 is vital for clinical researchers as it helps them navigate the complexities of device approval effectively. By adhering to these regulations, they can enhance their submissions and contribute to the advancement of medical technologies that improve patient outcomes.