


The article emphasizes the critical importance of mastering antibodies nomenclature for success in clinical research, underlining its essential role in therapeutic development and regulatory compliance.
By detailing how standardized naming conventions enhance clarity and collaboration among researchers, it illustrates how these practices prevent misclassification that could delay regulatory approvals.
Ultimately, this understanding facilitates the effective development and communication of immune therapies, reinforcing the need for precision in this complex field.
The intricate world of antibodies plays a pivotal role in clinical research, serving as the backbone for innovative therapies that combat diseases ranging from cancer to autoimmune disorders. Understanding the complexities of antibody nomenclature is essential for researchers aiming to navigate the rapidly evolving landscape of immune therapies.
However, with the introduction of new naming conventions and classifications, how can researchers ensure they are equipped to avoid costly misclassifications that could delay crucial treatments? This article delves into the significance of mastering antibody nomenclature, offering insights that could be the key to unlocking clinical research success.
Antibodies are specialized proteins produced by the immune system that identify and neutralize foreign entities such as bacteria and viruses. In clinical research, particularly in therapeutic development, these proteins are indispensable. They fulfill various roles, including diagnostics, targeted therapies, and as essential research tools. A comprehensive understanding of immune protein structure and function, including antibodies nomenclature, is crucial for researchers aiming to develop effective therapies while adhering to regulatory standards. Their unique ability to bind specifically to antigens underpins the creation of monoclonal antibodies (mAbs), which are designed for therapeutic applications in conditions such as cancer, autoimmune disorders, and infectious diseases, as indicated by antibodies nomenclature.
The global landscape of immune therapies has witnessed significant growth, with a total of 162 such treatments authorized worldwide as of June 30, 2022, underscoring their vital role in modern medicine. Therapeutic proteins now represent the fastest-growing category of biological medications, with a market projected to reach 445 billion USD by 2028, driven by a compound annual growth rate (CAGR) of 13.2% from 2022 to 2028. Notably, agents targeting PD-1 have revolutionized cancer treatment, with 12 approved therapies enhancing patient outcomes.
Case studies further illustrate the impact of immune proteins in therapeutic development. For instance, Rituximab, a monoclonal agent targeting CD20, has become a standard treatment for various blood cancers, demonstrating the effectiveness of mAbs in oncology. Similarly, Palivizumab, the only treatment approved for respiratory syncytial virus (RSV), has shown a 55% reduction in hospitalizations, emphasizing the clinical importance of immune therapies. In fact, approximately 78% of total immune response product sales in the US are attributed to cancer and autoimmune diseases, highlighting the financial significance of these therapies.
Experts in immunology emphasize the transformative potential of immune proteins in treatment. As Prerna Sharma notes, "In recent years, these immune proteins have emerged as the fastest-growing category of biological medications authorized for the treatment of a broad spectrum of illnesses, from cancer to autoimmune disorders." As the field continues to advance, the necessity for systematic data collection and analytics becomes increasingly apparent, ensuring that the benefits of mAbs are accurately evaluated and communicated. This thorough understanding of immune proteins not only propels therapeutic innovation but also informs health strategies and equitable access to groundbreaking treatments.

Antibodies nomenclature follows specific guidelines established by organizations such as the World Health Organization (WHO) and the United States Adopted Names (USAN) Council. This naming convention typically includes:
Notably, the suffix '-mab' is frequently utilized for monoclonal proteins. Recent updates have introduced new antibodies nomenclature rules to accommodate the growing diversity of immune protein formats, including bispecific formats and drug-conjugated proteins. Understanding these classifications is crucial for researchers as it facilitates the precise identification and discussion of various treatment therapies, thereby promoting collaboration and adherence in research studies.
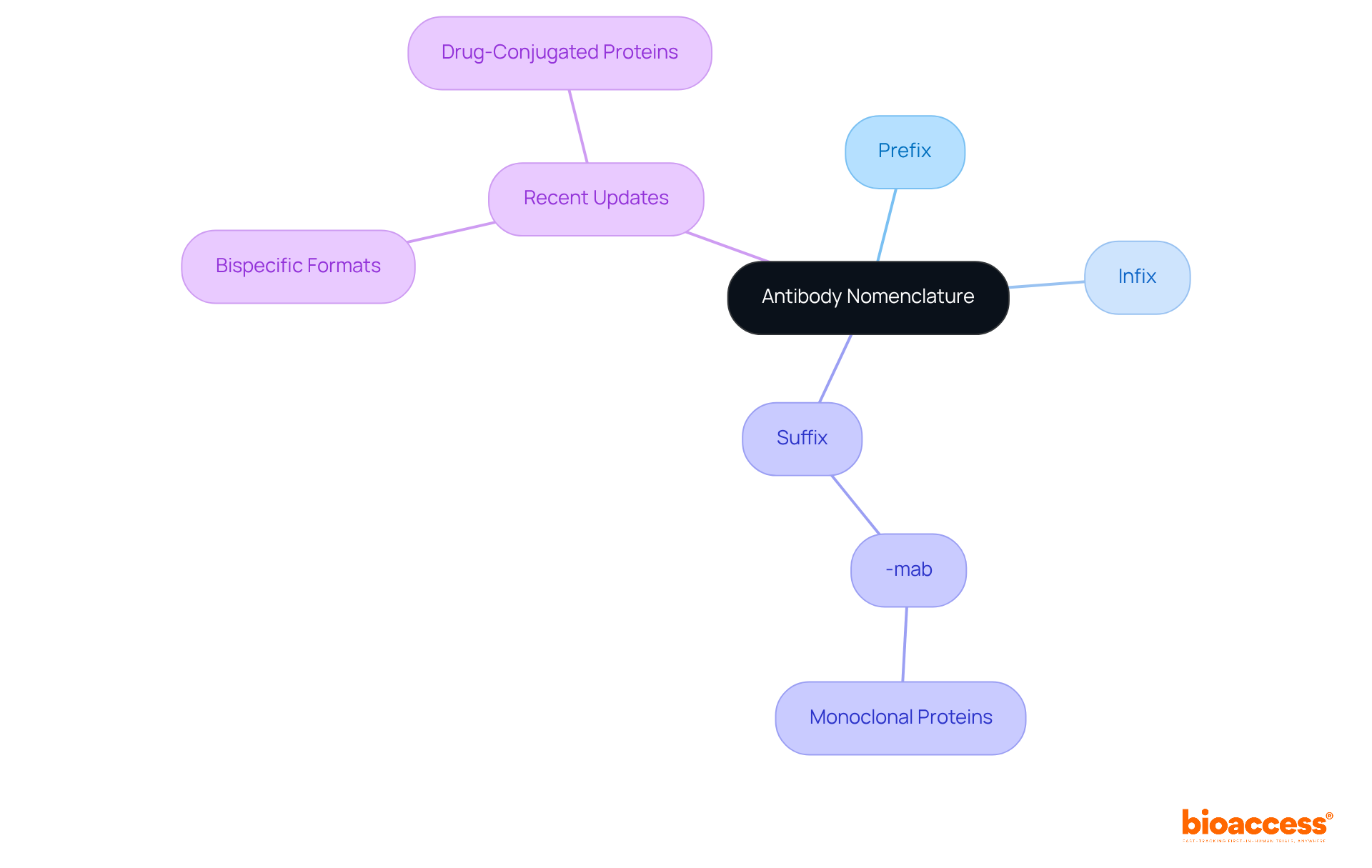
To effectively utilize antibodies nomenclature in medical research, scientists must adhere to established naming conventions when documenting their studies. This practice encompasses the precise use of suffixes and prefixes in all communications, publications, and regulatory submissions. For example, in the submission of a clinical trial application, employing the correct nonproprietary name of the therapeutic agent is essential to avoid confusion and ensure regulatory compliance.
Statistics reveal that improper antibodies nomenclature can lead to considerable delays in regulatory approvals; some studies indicate that misclassification can prolong approval timelines by several months. Furthermore, researchers must remain informed about updates in antibodies nomenclature guidelines, such as the elimination of the -mab suffix and the introduction of new suffixes like -tug, -bart, -mig, or -ment, as these changes can significantly influence the classification and approval of new therapeutic agents.
By utilizing standardized nomenclature, clarity is enhanced, fostering collaboration among researchers, sponsors, and regulatory bodies, ultimately contributing to the success of clinical trials. As Paul Carter emphasized, the assignment of a unique International Nonproprietary Name (INN) is crucial for the identification of pharmaceutical substances, and strict adherence to these naming conventions is vital to avoid misclassification and ensure a smoother pathway to market.

Understanding the intricacies of antibodies and their nomenclature is critical for success in clinical research. These specialized proteins play a pivotal role in therapeutic development, diagnostics, and targeted therapies, making their classification and naming conventions essential for researchers. Mastering antibodies nomenclature not only enhances communication among scientists but also ensures compliance with regulatory standards, ultimately facilitating the advancement of innovative treatments.
The article highlights key insights into the role of antibodies in modern medicine, illustrating their growth and significance in therapeutic applications. It emphasizes the importance of adhering to established naming conventions to avoid delays in regulatory approvals and enhance clarity in research documentation. By examining case studies and expert opinions, the article underscores the transformative impact of immune proteins, such as monoclonal antibodies, in treating various diseases, particularly cancer and autoimmune disorders.
In light of the rapid evolution of antibody therapies and nomenclature, it becomes increasingly vital for researchers to stay informed and implement best practices in their studies. By prioritizing accurate and standardized naming conventions, the clinical research community can promote collaboration, improve regulatory outcomes, and ultimately drive the development of groundbreaking therapies that can change patient lives. Embracing this knowledge is not just an academic exercise; it is a fundamental step toward achieving success in the ever-evolving landscape of clinical research.
What are antibodies and what role do they play in clinical research?
Antibodies are specialized proteins produced by the immune system that identify and neutralize foreign entities such as bacteria and viruses. In clinical research, they are crucial for therapeutic development, serving roles in diagnostics, targeted therapies, and as essential research tools.
Why is understanding antibody structure and function important for researchers?
A comprehensive understanding of immune protein structure and function, including antibodies nomenclature, is essential for researchers to develop effective therapies while adhering to regulatory standards.
What are monoclonal antibodies (mAbs) and their therapeutic applications?
Monoclonal antibodies (mAbs) are designed to bind specifically to antigens and are used in therapeutic applications for conditions such as cancer, autoimmune disorders, and infectious diseases.
How has the global landscape of immune therapies changed recently?
The global landscape of immune therapies has seen significant growth, with 162 treatments authorized worldwide as of June 30, 2022. Therapeutic proteins are the fastest-growing category of biological medications, projected to reach a market value of 445 billion USD by 2028.
What impact have agents targeting PD-1 had on cancer treatment?
Agents targeting PD-1 have revolutionized cancer treatment, with 12 approved therapies that have enhanced patient outcomes.
Can you provide examples of successful immune therapies?
Yes, Rituximab, a monoclonal agent targeting CD20, is a standard treatment for various blood cancers. Palivizumab, the only treatment approved for respiratory syncytial virus (RSV), has shown a 55% reduction in hospitalizations.
What is the financial significance of immune therapies in the US?
Approximately 78% of total immune response product sales in the US are attributed to cancer and autoimmune diseases, highlighting the financial importance of these therapies.
What is the future outlook for immune proteins in treatment?
Experts in immunology believe that immune proteins have transformative potential in treatment for a wide range of illnesses. Systematic data collection and analytics will be crucial for evaluating and communicating the benefits of mAbs as the field advances.