


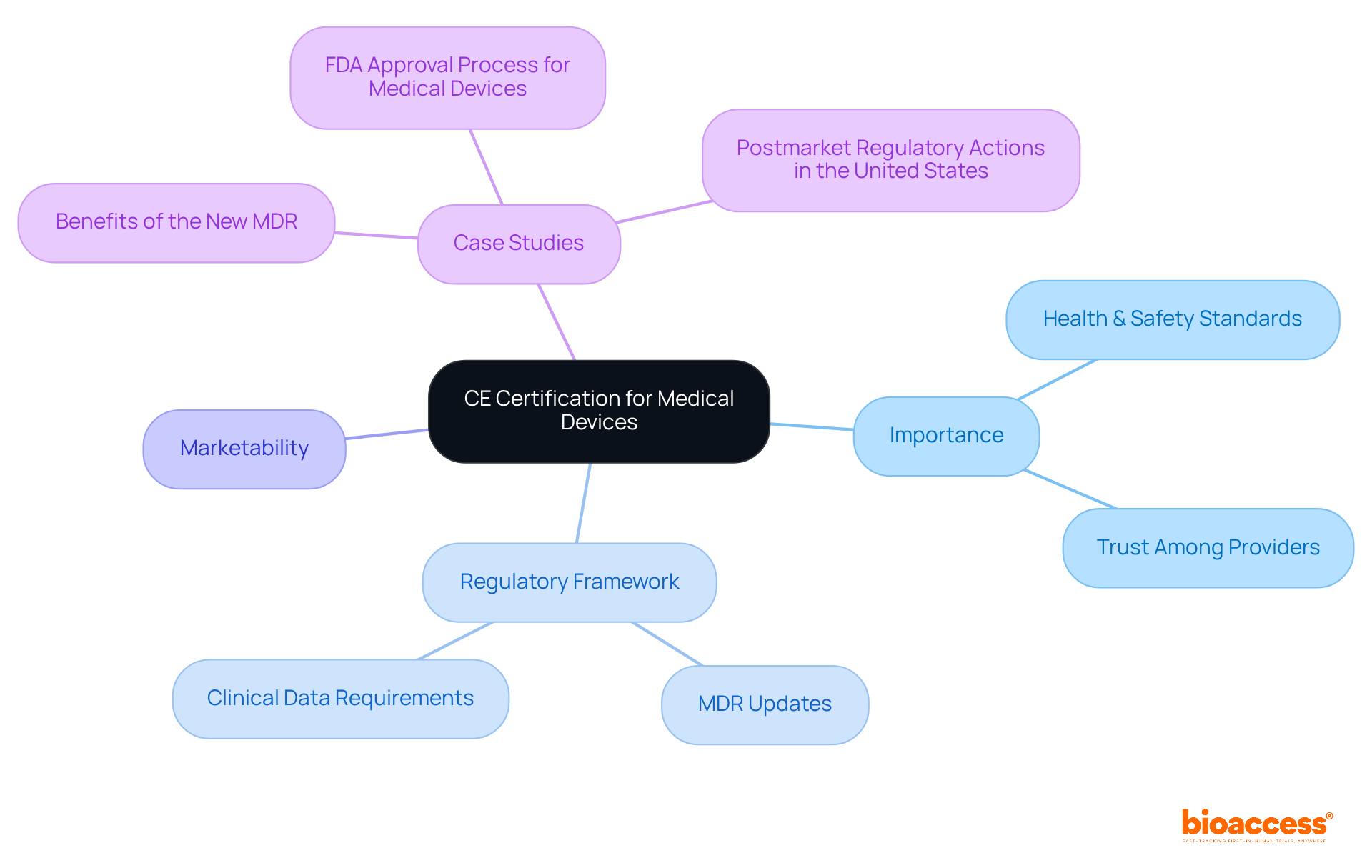
The article presents a thorough step-by-step guide on mastering CE certification for medical devices, underscoring its vital role in ensuring compliance with EU health, safety, and environmental standards.
It details the classification of medical devices and the CE marking process, emphasizing the significance of leveraging resources and expert guidance to navigate the intricacies of certification.
This underscores the necessity of adhering to regulatory frameworks to achieve successful market access in Europe.
The CE certification of medical devices serves as a crucial gateway for manufacturers seeking to penetrate the European market, ensuring adherence to rigorous health and safety regulations. This certification process not only bolsters the credibility of medical products but also profoundly influences their marketability and acceptance among healthcare providers and consumers.
However, the recent updates to the regulatory landscape have rendered the navigation of CE certification complexities increasingly challenging.
How can manufacturers adeptly classify their devices and streamline the certification process to ensure compliance while simultaneously maximizing their competitive edge?
The CE certification of medical devices is a crucial necessity for medical equipment intended for sale in the European Economic Area (EEA). This endorsement signifies that a product complies with the EU's stringent health, safety, and environmental protection standards. The presence of a CE mark significantly enhances the marketability of medical devices, fostering trust among healthcare providers and consumers regarding the product's safety and effectiveness. For manufacturers, understanding the implications of the CE certification of medical devices is vital, as it directly influences their ability to commercialize products in Europe and adhere to rigorous regulatory frameworks.
Recent updates to the CE certification of medical devices, particularly under the Medical Device Regulation (MDR), have introduced more rigorous evaluation methods. These changes emphasize the need for strong clinical data and post-market monitoring. Case studies illustrate that products lacking CE approval face market exclusion, underscoring the importance of compliance in maintaining a competitive edge and ensuring patient safety.
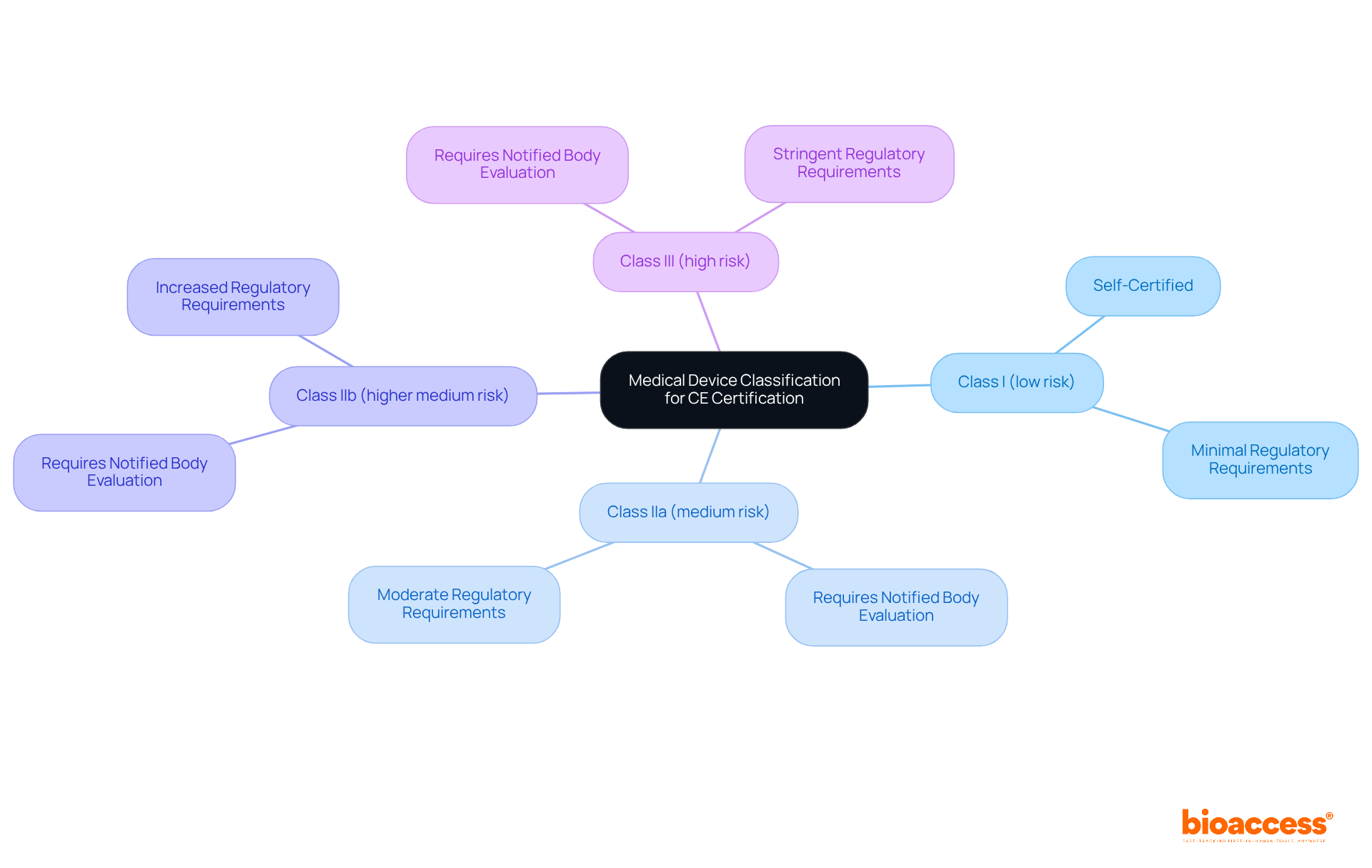
The procedure for CE certification of medical devices begins with the categorization of medical instruments in accordance with the EU Medical Device Regulation (MDR). Medical devices are classified into four distinct classes based on their risk levels:
Each class entails specific requirements and conformity assessment pathways necessary for CE certification of medical devices. Notably, Class I items may frequently be self-certified, whereas Class II and III products typically require evaluation by a Notified Body. A comprehensive understanding of these classifications and their respective assessment routes is vital for ensuring compliance and achieving successful CE certification of medical devices.
The CE certification of medical devices is a critical aspect of regulation, significantly influenced by the classification of the equipment and the complexity of the required documentation. For lower-risk equipment, this procedure may take as little as 4-6 weeks; however, for higher-risk equipment, it can extend to several months or even years. Key responsibilities during this process encompass:
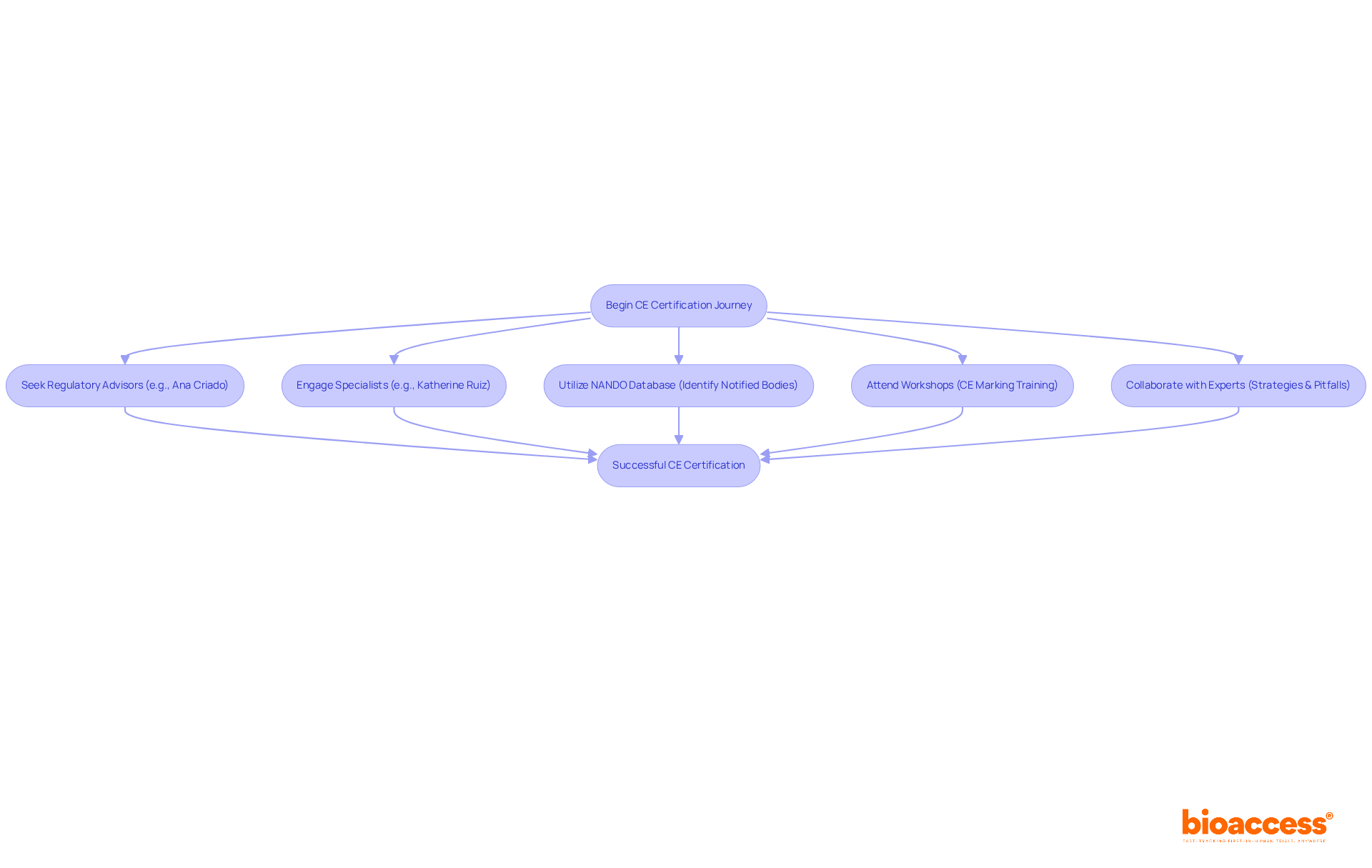
Manufacturers must prioritize clear communication with Notified Bodies and oversight authorities, such as INVIMA, the Colombian National Food and Drug Surveillance Institute, which plays an essential role in overseeing medical device regulation in Colombia. Engaging with specialists like Ana Criado, who possesses extensive experience in compliance matters and biomedical engineering, can empower manufacturers to navigate the complexities of adherence more effectively. Her insights into the Colombian compliance framework can facilitate a more streamlined approval process, ensuring that all requisite standards are met.

Producers pursuing the CE certification of medical devices should utilize various resources to enhance their chances of success. Regulatory advisors such as Ana Criado, the Director of Regulatory Affairs with extensive expertise in biomedical engineering and health economics, can provide critical guidance. Additionally, engaging with specialists like Katherine Ruiz, who focuses on Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, can yield invaluable insights into compliance requirements.
Utilizing tools such as the NANDO database is essential for identifying the appropriate Notified Bodies for approval. Furthermore, attending workshops and training sessions centered on CE marking will bolster understanding and preparedness. Collaborating with seasoned experts in the field can also illuminate optimal strategies and common pitfalls to avoid during the credentialing process.
To successfully navigate the CE certification of medical devices journey, manufacturers must actively seek these resources and consider forming partnerships with regulatory experts.
The journey to obtaining CE certification for medical devices stands as a regulatory necessity and a crucial milestone that profoundly influences marketability and consumer confidence. This certification guarantees that medical products adhere to the EU's stringent health, safety, and environmental standards, thus protecting patients and bolstering manufacturers' competitive advantage within the European market.
The article delved into essential facets of the CE certification process, highlighting the significance of:
Special attention was directed towards the dynamic regulatory environment, particularly under the Medical Device Regulation (MDR), which demands comprehensive clinical data and continuous post-market surveillance. Furthermore, the importance of collaborating with regulatory experts and leveraging available resources was underscored as a strategy to facilitate the certification journey.
In conclusion, the importance of CE certification transcends mere compliance; it embodies a dedication to quality and safety within the healthcare domain. Manufacturers are urged to actively seek guidance, utilize available resources, and stay abreast of regulatory developments to adeptly navigate the CE certification landscape. By embracing these proactive steps, they not only amplify their market presence but also contribute to the overarching objective of enhancing patient outcomes through the provision of safe and effective medical devices.
What is CE certification for medical devices?
CE certification for medical devices is an endorsement that indicates a product complies with the European Union's health, safety, and environmental protection standards, allowing it to be sold in the European Economic Area (EEA).
Why is CE certification important for medical devices?
CE certification is important because it enhances the marketability of medical devices, builds trust among healthcare providers and consumers regarding safety and effectiveness, and is essential for manufacturers to commercialize their products in Europe.
What recent updates have been made to CE certification under the Medical Device Regulation (MDR)?
Recent updates to CE certification under the MDR have introduced more rigorous evaluation methods, emphasizing the need for strong clinical data and post-market monitoring.
What are the consequences of not having CE approval for medical devices?
Products lacking CE approval face market exclusion, which highlights the importance of compliance for maintaining a competitive edge and ensuring patient safety.
How does CE certification impact manufacturers of medical devices?
CE certification impacts manufacturers by influencing their ability to commercialize products in Europe and requiring adherence to rigorous regulatory frameworks.