


CE marking is essential for medical devices sold in the European Economic Area. It signifies compliance with EU safety and health regulations, enabling market access and ensuring consumer confidence. This article outlines a step-by-step process for obtaining CE marking. It emphasizes the importance of:
By doing so, it effectively guides manufacturers in navigating the certification process.
Navigating the intricate landscape of medical device regulation presents significant challenges, particularly in grasping the critical importance of CE marking. This vital certification not only facilitates market access within the European Economic Area but also instills confidence in consumers regarding the safety and efficacy of medical products. As manufacturers endeavor to comply with rigorous EU standards, they face a multitude of obstacles—ranging from classification and documentation to compliance assessments and post-market responsibilities.
How can they adeptly navigate the CE marking process to ensure their devices not only meet compliance but also excel in a competitive marketplace?
CE certification is a critical approval for a CE mark medical device sold in the European Economic Area (EEA). It signifies compliance with EU safety, health, and environmental protection requirements. Understanding its significance means recognizing that CE certification not only facilitates entry into the European market but also assures consumers and healthcare professionals of the product's safety and effectiveness. Without the CE mark medical device approval, a medical product cannot be legally marketed in the EU, making it essential for producers aiming to penetrate this lucrative market. Furthermore, CE certification enhances a company's reputation, demonstrating a commitment to quality and compliance with stringent regulations.
Experts like Ana Criado, Director of Regulatory Affairs, emphasize the vital role of CE labeling in ensuring that medical devices meet necessary compliance standards. With over five years of experience at Colombia’s regulatory agency—INVIMA—and her consulting work for global companies, Ana's insights highlight the importance of grasping these compliance requirements.
In summary, CE marking is crucial for:
By understanding these elements, manufacturers can navigate the complexities of the CE certification process more effectively.

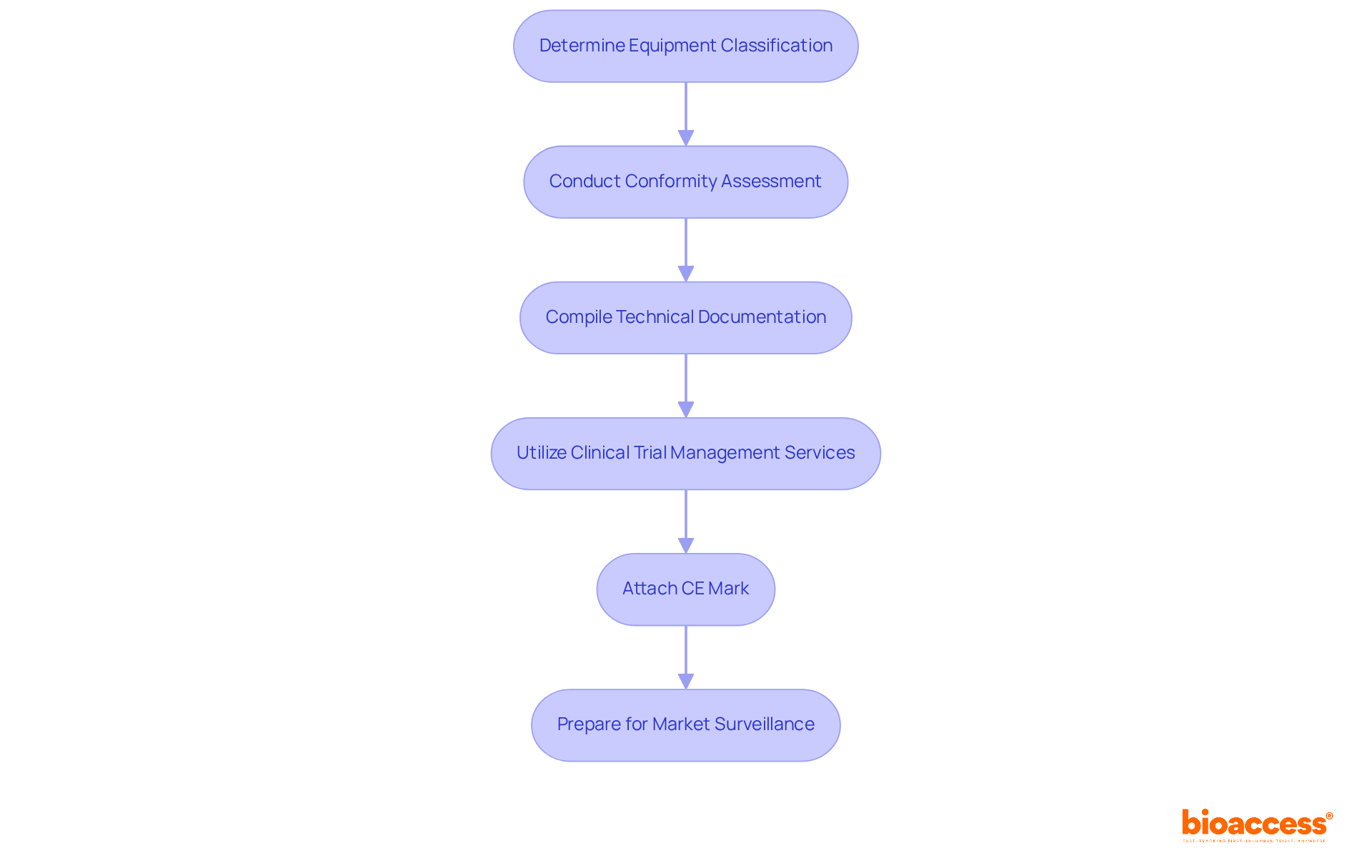
To obtain CE marking for your medical device, it is imperative to follow these steps:
Determine the Classification of Your Equipment: Medical instruments are categorized into four groups (Class I, IIa, IIb, III) based on risk. Understanding your equipment's classification is essential as it dictates the regulatory pathway.
Conduct a Conformity Assessment: Depending on the classification, you may need to perform a self-assessment or engage a Notified Body for a more rigorous evaluation. This assessment confirms that your equipment meets the necessary safety and performance standards.
Compile Technical Documentation: Prepare a technical file that includes design specifications, risk assessments, and clinical evaluations. This documentation is crucial for demonstrating adherence to EU regulations.
Utilize Comprehensive Clinical Trial Management Services: Collaborate with professionals such as Katherine Ruiz, who specializes in compliance matters for medical instruments and in vitro diagnostics in Colombia. These services encompass feasibility studies, site selection, regulatory reviews, trial setup, import permits, study project management, monitoring, and reporting, all of which are vital for ensuring that your clinical trials meet regulatory requirements.
Attach the CE mark to your product: After confirming adherence, you can attach the CE mark to your product, indicating that it meets all relevant EU requirements.
Prepare for Market Surveillance: Following the acquisition of CE certification, establish a post-market surveillance system to monitor the device's performance and safety in the market.
By following these steps and utilizing extensive clinical trial management services, including review and feedback on study documents, manufacturers can systematically approach the CE certification process, ensuring compliance and facilitating market entry.
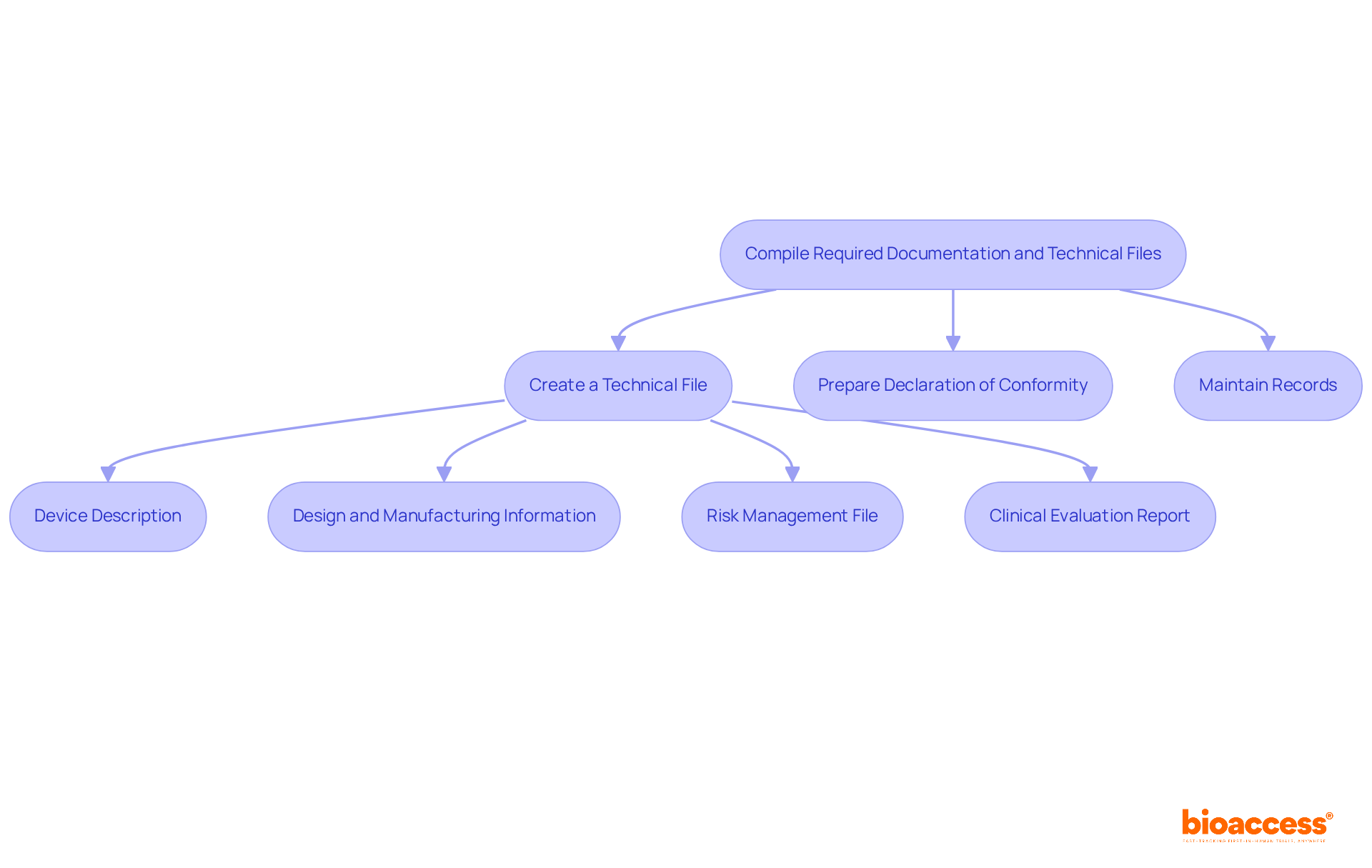
Compiling the required documentation and technical files is a vital part of the CE mark medical device process. Here’s how to do it:
Create a Technical File: This file should include:
Prepare Declaration of Conformity: This document states that your device complies with all applicable EU directives and regulations. It must be signed by the manufacturer or their authorized representative.
Maintain Records: Keep all documentation organized and accessible for review by Notified Bodies or oversight authorities. This includes records of design changes, quality control measures, and post-market surveillance activities.
In addition to these steps, engaging with comprehensive clinical trial management services, such as those offered by bioaccess, can significantly streamline the process. Services such as feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting are vital for ensuring that all legal requirements are met efficiently. By carefully assembling these documents and utilizing expert services, manufacturers can guarantee a smoother process for the CE mark medical device and demonstrate their dedication to quality and standards.
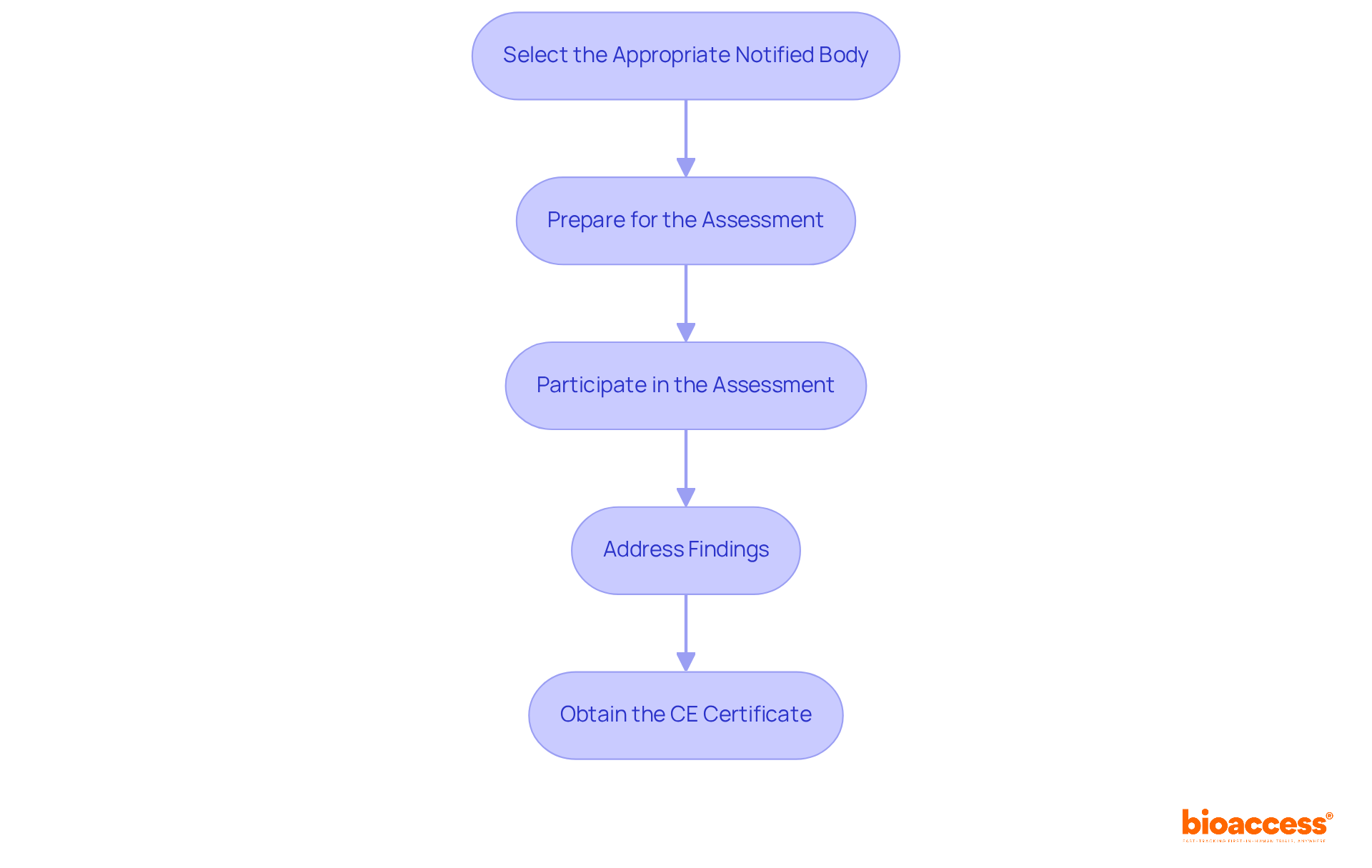
Interacting with Notified Bodies is essential for the compliance evaluation of various medical products. To navigate this process effectively, consider the following steps:
Select the Appropriate Notified Body: Choose a Notified Body that is accredited for your specific type and classification. Evaluate their expertise, reputation, and experience in your product category.
Prepare for the Assessment: Prior to the assessment, ensure that all documentation is complete and organized. Conduct internal audits to identify and address any potential regulatory gaps.
Participate in the Assessment: During the assessment, be ready to answer questions and provide additional information as needed. This may include on-site inspections and discussions regarding your quality management system.
Address Findings: If the Notified Body identifies any non-conformities, promptly address these issues and provide evidence of the corrective actions taken.
Obtain the CE Certificate: Once adherence is confirmed, the Notified Body will issue a CE certificate, enabling you to affix the CE mark to your apparatus.
By effectively engaging with Notified Bodies, manufacturers can ensure a thorough conformity evaluation, facilitating a smoother route to market entry.
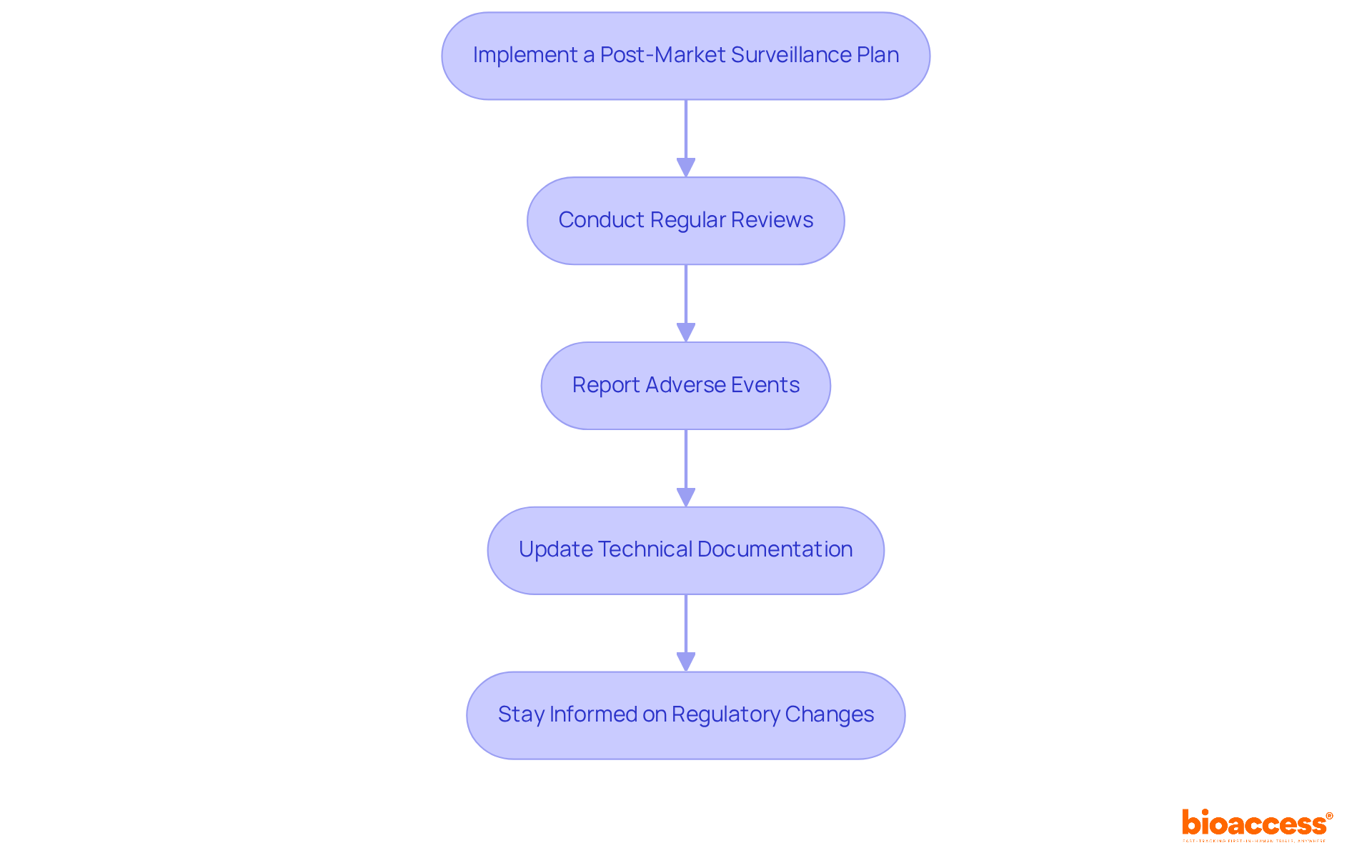
Maintaining compliance with the CE mark medical device requirements and understanding post-market obligations are crucial for the long-term success of your medical product. Here’s what you need to do:
Implement a Post-Market Surveillance Plan: Create a strategy to oversee the product's performance and safety in the market. This includes collecting data on adverse events and user feedback, supported by bioaccess's comprehensive clinical trial management services.
Conduct Regular Reviews: Periodically examine the post-market data to identify trends or issues that may necessitate corrective actions or updates to the product. Engaging with regulatory affairs experts like Katherine Ruiz and Ana Criado can provide valuable insights during these reviews.
Report Adverse Events: Establish a system for reporting adverse events to the relevant authorities as required by EU regulations. This is essential for maintaining transparency and ensuring patient safety, facilitated through effective project management and monitoring services offered by bioaccess.
Update Technical Documentation: Keep your technical documentation up to date with any changes made to the device or its manufacturing process. This involves updating the risk management document and clinical evaluation report as needed, guaranteeing adherence to country requirements as outlined by bioaccess's services, including feasibility studies and site selection.
Stay Informed on Regulatory Changes: Regularly review updates to EU regulations and guidelines to ensure ongoing adherence. Connect with industry associations or governing organizations to remain updated on best practices. Utilizing the knowledge of experts such as Ana Criado, who possesses substantial experience in regulatory matters, can be advantageous.
By maintaining compliance and understanding post-market obligations, manufacturers can ensure the continued safety and efficacy of their CE mark medical devices, thereby protecting patients and enhancing their market reputation.
CE marking is a fundamental requirement for medical devices seeking entry into the European market, signifying compliance with EU regulations on safety, health, and environmental protection. This certification not only facilitates access to a lucrative market but also instills confidence in consumers and healthcare professionals regarding the product's safety and effectiveness. Understanding the significance of CE marking is essential for manufacturers who wish to navigate the complexities of regulatory compliance and enhance their brand reputation.
The article outlines a comprehensive step-by-step process to obtain CE marking, including:
Each of these steps plays a critical role in ensuring that medical devices meet the required safety and performance standards, ultimately leading to successful market entry. Furthermore, maintaining compliance through post-market obligations is vital for the long-term success of these products, ensuring ongoing safety and effectiveness.
In conclusion, the importance of CE marking extends beyond mere regulatory compliance; it represents a commitment to quality and safety that benefits both manufacturers and end-users. By following the outlined steps and understanding the regulatory landscape, manufacturers can effectively position their medical devices in the market while safeguarding patient health. Engaging with experts and utilizing comprehensive clinical trial management services can further streamline this process, ensuring that all legal requirements are met efficiently.
What is CE marking and why is it important for medical devices?
CE marking is a certification that signifies a medical device's compliance with EU safety, health, and environmental protection requirements. It is essential for market access in the European Economic Area (EEA), assuring consumers and healthcare professionals of the product's safety and effectiveness.
What are the consequences of not having CE marking for a medical device?
Without CE marking, a medical device cannot be legally marketed in the EU, which is crucial for producers aiming to enter this significant market.
How does CE marking enhance a company's reputation?
CE marking demonstrates a company's commitment to quality and compliance with stringent regulations, which can enhance its reputation in the industry.
What are the main benefits of CE marking for manufacturers?
The main benefits include market access to the EU, increased consumer confidence in product safety and performance, and assurance of regulatory compliance, which reduces the risk of legal issues.
What is the first step to obtain CE marking for a medical device?
The first step is to determine the classification of the medical device, as it is categorized into four groups (Class I, IIa, IIb, III) based on risk, which dictates the regulatory pathway.
What does the conformity assessment involve?
Depending on the classification, the conformity assessment may require a self-assessment or engagement with a Notified Body for a rigorous evaluation to confirm that the device meets necessary safety and performance standards.
What technical documentation is required for CE marking?
Manufacturers must compile a technical file that includes design specifications, risk assessments, and clinical evaluations to demonstrate adherence to EU regulations.
How can clinical trial management services assist in the CE marking process?
Clinical trial management services help with feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, monitoring, and reporting, ensuring that clinical trials meet regulatory requirements.
What should manufacturers do after obtaining CE marking?
Manufacturers should establish a post-market surveillance system to monitor the device's performance and safety in the market following the acquisition of CE certification.