


The article delineates four pivotal strategies for mastering clinical development:
Each strategy is underpinned by practical approaches, including:
These strategies ultimately lead to improved study outcomes and expedited approvals, underscoring their significance in the clinical research landscape.
Navigating the intricate landscape of clinical development necessitates a strategic approach to ensure success in an increasingly competitive environment. By mastering four pivotal strategies—effective regulatory navigation, innovative patient recruitment, leveraging diverse patient pools, and adopting cutting-edge technologies—research teams can significantly enhance their operational efficiency and improve study outcomes.
However, as the regulatory landscape evolves and patient expectations shift, organizations must consider:
Navigating regulatory frameworks effectively, particularly in Colombia, necessitates staying informed about the specific rules governing trials in each sector. It is essential to comprehend the requirements established by local regulatory bodies, including the Institutional Review Board (IRB) or Ethics Committee (EC), INVIMA (the regulatory agency), and the Ministry of Industry and Commerce (MinCIT) for import permits. A proactive approach is imperative and involves:
By implementing these strategies, teams involved in clinical development can significantly minimize the risk of non-compliance and expedite the approval process, ultimately leading to faster study initiation and completion.
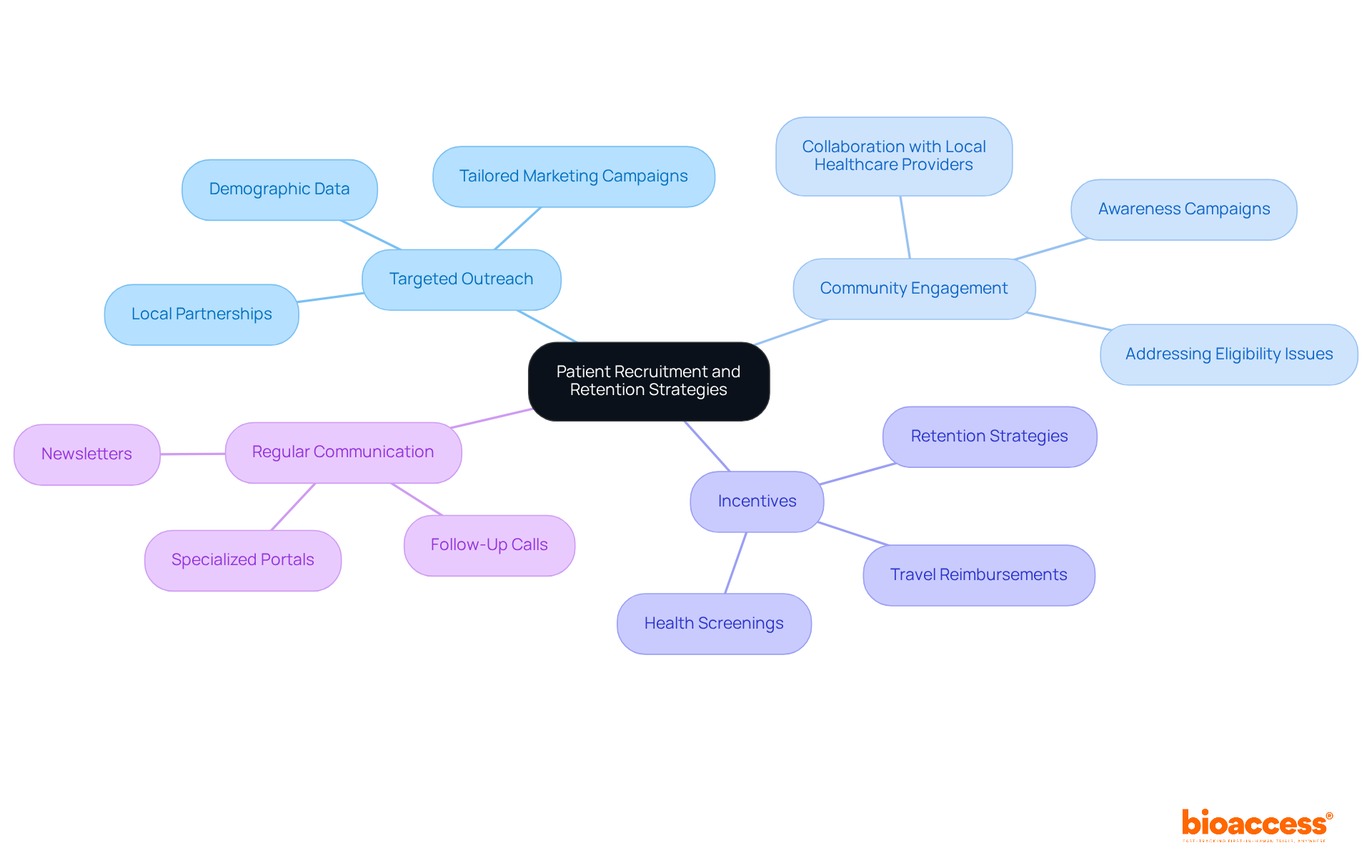
To enhance patient recruitment and retention, it is essential to implement effective strategies that resonate with the target audience.
Targeted Outreach: Utilize demographic data to identify and engage potential participants through tailored marketing campaigns. This approach proves particularly effective in regions like Colombia, where partnerships with local organizations can facilitate access to diverse patient populations.
Community Engagement: Collaborate with local healthcare providers and community organizations to increase awareness about the research and its advantages. Such collaborations can help overcome the reluctance of potential participants and address eligibility issues that often hinder recruitment efforts.
Incentives: Offer incentives for participation, such as travel reimbursements or health screenings, to encourage enrollment and retention. This strategy has demonstrated effectiveness in increasing subject retention rates, as evidenced by successful partnerships like that of GlobalCare Clinical Trials and bioaccess, which achieved over 95% retention.
Additionally, maintaining regular communication with participants throughout the study fosters trust and commitment, leading to improved retention rates. This can be accomplished through follow-up calls, newsletters, or specialized portals that offer updates and resources. By implementing these strategies, research directors can significantly enhance their recruitment and retention efforts, particularly in challenging environments.
To effectively leverage diverse patient pools for clinical development, consider the following strategies:
Geographic Diversity: Conduct trials across multiple regions, including Latin America, where collaborations like that of bioaccess™ and Caribbean Health Group are paving the way for Barranquilla to become a leading destination for clinical trials. This method not only improves enrollment rates but also guarantees that research outcomes are more reflective of the global population.
Cultural Competence: Equip your team with training to understand and respect cultural differences. This fosters better communication and builds trust with potential participants, which is crucial for successful recruitment.
Collaborative Networks: Establish partnerships with local clinics and hospitals that serve diverse communities. Partnerships, like those backed by Colombia's Minister of Health, can enhance outreach and boost recruitment efforts, ensuring that under-engaged groups are involved in research studies.
Embracing diversity in patient recruitment not only speeds up enrollment but also improves the relevance of research findings for clinical development across a wider demographic, ultimately increasing the influence of studies. For example, organizations such as the Lazarex Cancer Foundation have effectively enhanced access to research studies for underserved populations by covering travel expenses and offering navigation support, showcasing the concrete advantages of community involvement.

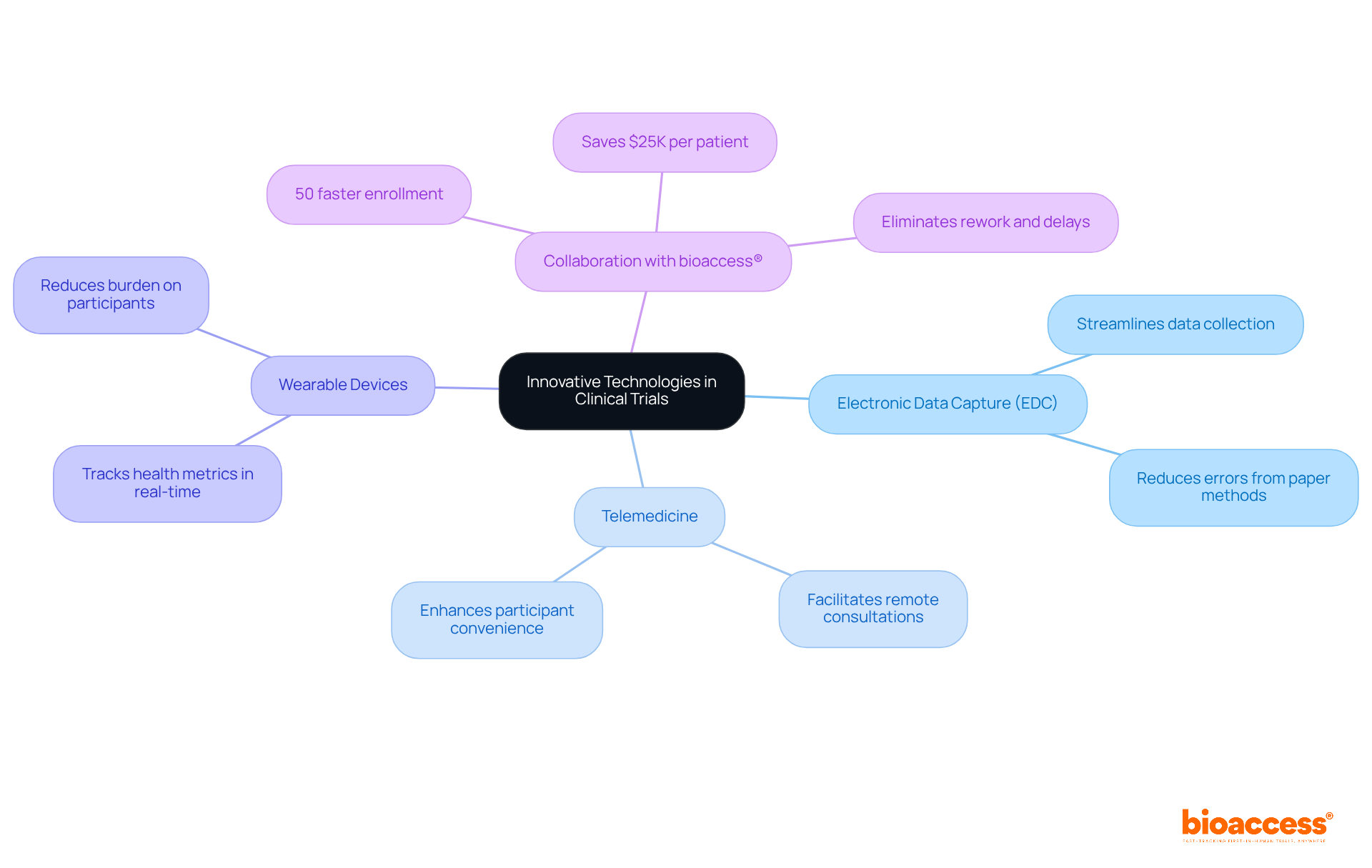
Incorporating innovative technologies into clinical development can significantly enhance their efficiency and effectiveness. Key technologies to consider include:
Alongside these technologies, collaborating with bioaccess® can further expedite your research studies. With the ability to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, bioaccess® offers FDA-ready data that saves $25K per patient, eliminating rework and delays. Their extensive trial management services include feasibility assessments, site selection, compliance evaluations, trial preparation, import permits, project oversight, and reporting. By adopting these technologies and leveraging bioaccess®'s expertise, teams involved in clinical development can improve operational efficiency, enhance participant experience, and ultimately drive better study outcomes.
Mastering clinical development hinges on the implementation of four essential strategies that drive success. By focusing on:
organizations can significantly improve their clinical trial processes and outcomes.
The importance of understanding local regulations, engaging with regulatory authorities, and maintaining thorough documentation cannot be overstated; these elements ensure compliance and expedite study approvals. Effective patient recruitment and retention strategies, such as targeted outreach, community engagement, and the use of incentives, are crucial for building trust and commitment among participants. Furthermore, embracing geographic and cultural diversity in patient recruitment accelerates enrollment and enhances the relevance of research findings. Finally, integrating innovative technologies like electronic data capture, telemedicine, and wearable devices streamlines operations and improves participant experiences.
In reflection, the strategies discussed serve as a roadmap for organizations aiming to enhance their clinical development efforts. By prioritizing compliance, fostering community relationships, embracing diversity, and leveraging technology, stakeholders can navigate the complexities of clinical trials more effectively. Proactive steps in these areas lead to faster and more efficient studies and contribute to the advancement of medical knowledge that benefits a broader population. Embrace these strategies to ensure the success of clinical development initiatives in the ever-evolving landscape of healthcare research.
Why is it important to navigate regulatory frameworks in clinical trials?
Navigating regulatory frameworks is crucial for ensuring compliance with specific rules governing trials, which can minimize the risk of non-compliance and expedite the approval process.
What are the key regulatory bodies involved in clinical trials in Colombia?
The key regulatory bodies in Colombia include the Institutional Review Board (IRB) or Ethics Committee (EC), INVIMA (the regulatory agency), and the Ministry of Industry and Commerce (MinCIT) for import permits.
What proactive approaches can teams take to navigate regulatory frameworks effectively?
Teams can take several proactive approaches, including regular training to stay updated on regulations, engaging with regulatory authorities for clarification and guidance, and maintaining meticulous documentation of all regulatory submissions and communications.
How does regular training benefit teams involved in clinical development?
Regular training ensures that team members are well-versed in the latest regulations, which is essential for maintaining compliance and facilitating the approval process.
What is the role of documentation practices in regulatory compliance?
Maintaining meticulous records of all regulatory submissions and communications is essential for ensuring transparency and accountability, which helps in minimizing compliance risks.
What is the ultimate goal of effectively navigating regulatory frameworks in clinical trials?
The ultimate goal is to lead to faster study initiation and completion by minimizing compliance risks and expediting the approval process.