


The article highlights the essential strategies for effectively implementing Risk-Based Monitoring (RBM) in clinical trials. It asserts that RBM significantly enhances patient safety, reduces costs, and improves data integrity by focusing monitoring efforts on high-risk areas. This approach is bolstered by real-time data analysis and customized monitoring plans, ultimately transforming clinical study management for superior outcomes.
Clinical trial risk-based monitoring (RBM) is revolutionizing the clinical research landscape by prioritizing patient safety and data integrity through a strategic oversight approach. This innovative methodology not only enhances the efficiency of clinical studies but also promises significant cost savings and expedited access to groundbreaking treatments.
However, as organizations adopt RBM, they frequently encounter challenges that can impede its successful implementation.
How can clinical trial sponsors effectively navigate these complexities to fully leverage the advantages of risk-based monitoring and ensure their studies uphold the highest standards of quality and compliance?
Clinical trial risk based monitoring (RBM) represents a revolutionary approach to clinical study supervision, focusing on the detection and mitigation of threats that could jeopardize patient safety and data integrity. Unlike traditional oversight methods, which often rely on extensive on-site inspections and comprehensive source verification, RBM strategically directs monitoring efforts based on the specific risks associated with each study. This targeted strategy enables sponsors to allocate resources more efficiently, concentrating on critical areas that require closer scrutiny while minimizing oversight in lower-risk sectors.
As we look towards 2025, the adoption of clinical trial risk based monitoring is gaining significant traction, with studies revealing that approximately 88% of clinical trials now integrate at least one clinical trial risk based monitoring component. This shift underscores a growing recognition of the necessity for adaptive monitoring strategies, such as clinical trial risk based monitoring, that can effectively address the complexities inherent in modern clinical research. By leveraging real-time information analysis, clinical trial risk based monitoring not only improves the quality of clinical studies but also accelerates study completion times, ultimately facilitating quicker access to innovative treatments for patients.
Successful examples of clinical trial risk based monitoring implementation are evident across various therapeutic areas, where sponsors have employed Key Risk Indicators (KRIs) and centralized monitoring tools to proactively identify potential issues. This methodology has proven particularly effective in high-enrollment studies, where a single site can generate thousands of pages of data every few weeks, making conventional verification methods impractical. By focusing on essential data components rather than exhaustive validation, RBM has the potential to reduce clinical study costs by as much as 30%, while simultaneously improving data quality and operational efficiency.
However, common pitfalls in the implementation of clinical trial risk based monitoring include insufficient training for staff on RBM principles and a lack of clear communication channels among stakeholders. Addressing these challenges is crucial for maximizing the advantages of RBM.
As the clinical study landscape continues to evolve, the adoption of clinical trial risk based monitoring methodologies becomes imperative for safeguarding data integrity and ensuring timely submissions. The strategic foresight and operational expertise required to implement RBM effectively can transform operational constraints into opportunities for enhanced management, ultimately benefiting both sponsors and patients alike.
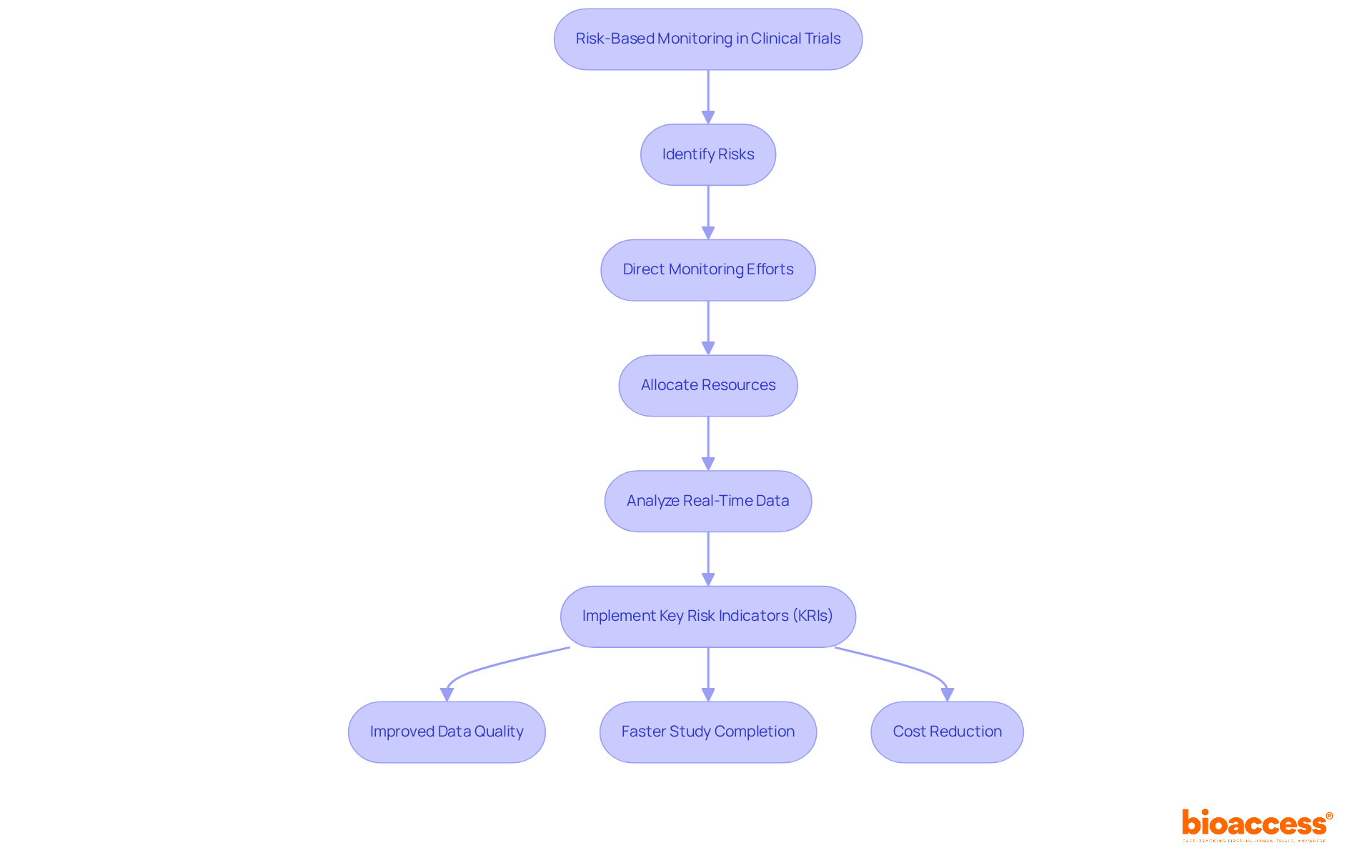
Clinical trial risk based monitoring plays a pivotal role in enhancing the efficiency and effectiveness of clinical studies. Its key benefits are numerous:
Enhanced Patient Safety: By concentrating on high-risk areas, RBM prioritizes patient safety, significantly reducing the likelihood of adverse events during studies. Bioaccess's structured project management ensures that these high-risk areas are meticulously monitored.
Cost Efficiency: RBM minimizes the necessity for frequent on-site monitoring visits, which can constitute a substantial portion of research costs. This strategic resource allocation empowers sponsors to optimize their budgets effectively. Bioaccess facilitates this efficiency through comprehensive feasibility studies and site selection.
Information Integrity: Focusing observation efforts on critical information points elevates the quality of the collected data, resulting in more reliable and credible study outcomes. Compliance evaluations conducted by Bioaccess bolster data integrity throughout the testing process.
Faster Study Completion: The streamlined approach of RBM accelerates timelines, enabling quicker access to innovative treatments for patients. Bioaccess's expertise in study setup and approval processes further supports this accelerated timeline.
Regulatory Compliance: RBM is aligned with regulatory guidelines from authorities such as the FDA and ICH, ensuring that studies meet necessary compliance standards while adopting innovative monitoring practices. Bioaccess's thorough reviews and feedback on study documents reinforce adherence to country requirements, emphasizing the importance of regulatory compliance.
While the advantages of clinical trial risk based monitoring are substantial, it is crucial to recognize the challenges associated with its implementation. Factors such as inertia and perceived uncertainties can hinder the adoption of RBM practices. Addressing these barriers is vital for stakeholders to fully leverage the benefits of this innovative approach. Additionally, the impact of Medtech clinical studies on local economies—through job creation, economic growth, healthcare enhancement, and international collaboration—further underscores the importance of effective clinical study management in the broader context of healthcare advancement.
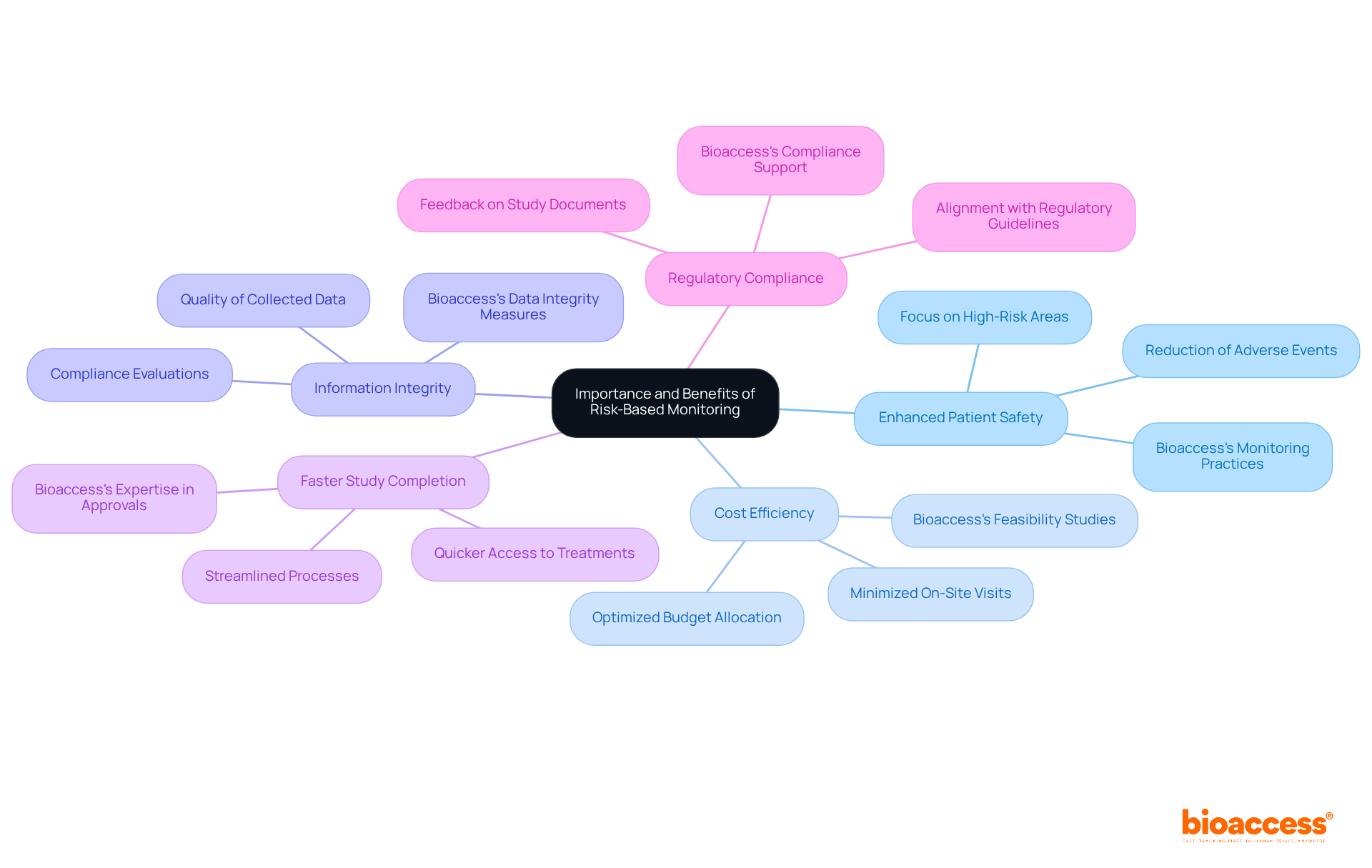
To implement effective Risk-Based Monitoring strategies, it is essential to consider the following steps:
Threat Evaluation: Begin with a comprehensive threat evaluation at the onset of the study to identify potential hazards associated with the trial. This evaluation should encompass the complexity of the protocol, the patient population, and the investigational product as part of clinical trial risk based monitoring. As Mark Zuckerberg aptly stated, 'The greatest danger is not taking any chance,' underscoring the importance of proactive threat management.
Customized Monitoring Plans: Develop monitoring plans tailored to the specific threats identified. This may necessitate adjustments in the frequency of monitoring visits and the types of information to be scrutinized under clinical trial risk based monitoring. It is crucial to avoid common pitfalls by ensuring that the monitoring plan remains flexible enough to adapt to unforeseen challenges.
Real-Time Data Utilization: Leverage real-time data analysis for clinical trial risk based monitoring to track trial progression and swiftly identify emerging challenges. This approach allows for proactive adjustments to the monitoring strategy as required. For instance, a local manufacturing company regained profitability after addressing threats, illustrating the tangible benefits of effective threat management.
Training and Communication: Ensure that all team members are well-versed in clinical trial risk based monitoring principles and the specific monitoring plan. Foster open communication among stakeholders to enhance collaboration and promptly address any issues that arise. Incorporating quotes from industry leaders during training sessions can further emphasize the significance of these management strategies.
Continuous Evaluation: Regularly assess the effectiveness of the clinical trial risk based monitoring strategy throughout the study. This involves evaluating whether the monitoring efforts adequately address the identified challenges and making necessary adjustments. Drawing parallels to the exemplary management techniques in Formula 1 can provide valuable insights into maintaining high standards in clinical studies.
By adhering to these best practices, clinical study teams can enhance their management capabilities, ensuring a more efficient and effective process. The anticipated outcome of implementing these RBM strategies includes improved study results and a more streamlined approach to risk management, ultimately contributing to the success of clinical research.
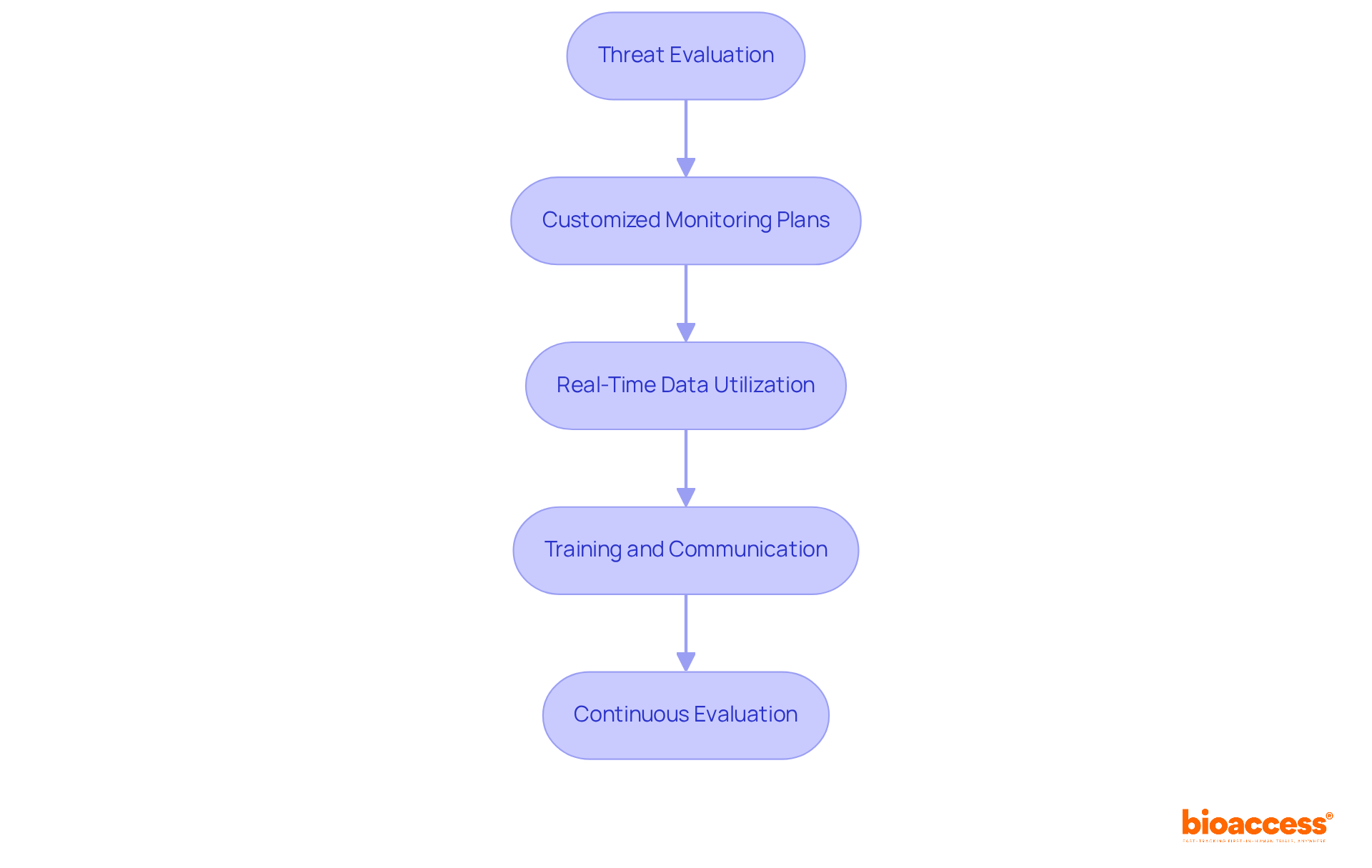
Leveraging technology is essential for enhancing Risk-Based Monitoring (RBM) in clinical trials. This section outlines several key tools and technologies that are pivotal in this endeavor:
Electronic Information Capture (EDC) Systems: These systems enhance information collection and management, enabling real-time access and monitoring. EDC systems significantly improve data accuracy by reducing human errors through automated entry processes and validation checks, ensuring compliance with regulatory standards. Notably, the EDC adoption rate in Canadian clinical studies is estimated at 41%, underscoring the increasing reliance on these systems.
Risk Assessment Tools: Implement software solutions designed to assist in conducting thorough risk assessments and tracking identified risks throughout the study. These tools are instrumental in proactively managing potential issues before they escalate.
Centralized Monitoring Platforms: Utilize platforms that integrate information from multiple sources, offering comprehensive oversight and analysis of trial data. This centralized approach enhances the ability to identify trends and anomalies in real-time, thereby improving decision-making.
Automated Reporting Tools: Employ tools that generate insights and alerts based on real-time data analysis. Automated reporting facilitates quicker decision-making and enhances responsiveness to emerging challenges. A recent survey revealed that 48.9% of respondents cited software cost as a barrier to EDC adoption, highlighting the necessity for cost-effective solutions.
Collaboration Platforms: Enhance communication among team members and stakeholders with collaboration tools. These platforms ensure alignment on monitoring strategies and findings, fostering a cohesive approach to risk management. Dr. John Smith, a clinical study specialist, emphasizes that EDC systems enhance information accuracy and overall study effectiveness, making them indispensable in contemporary clinical research.
By integrating these technologies into the RBM framework, clinical trial sponsors can significantly enhance their monitoring capabilities, leading to improved trial outcomes and increased patient safety. For instance, the implementation of an EDC system at the University of Missouri has enabled researchers to collect, store, and manage research data electronically in a secure and HIPAA-compliant repository, significantly reducing research-related time and costs.
The adoption of Risk-Based Monitoring (RBM) in clinical trials marks a transformative shift in the oversight of studies, emphasizing a strategic focus on identifying and mitigating risks to enhance patient safety and data integrity. By prioritizing high-risk areas and employing targeted monitoring efforts, RBM enables sponsors to allocate resources more effectively, ultimately leading to improved study outcomes and expedited access to innovative treatments.
Key insights from the discussion underscore the numerous advantages of RBM, including:
Effective implementation strategies—such as threat evaluation, customized monitoring plans, and leveraging technology—are crucial for overcoming common challenges in adopting RBM. Furthermore, the integration of advanced tools like Electronic Data Capture systems and centralized monitoring platforms significantly enhances the monitoring process, ensuring timely and accurate data management.
In reflection, the importance of embracing Risk-Based Monitoring transcends operational efficiencies; it embodies a commitment to advancing clinical research and improving patient outcomes. Stakeholders are urged to champion the adoption of RBM practices, invest in necessary training and technology, and cultivate a culture of proactive risk management. By taking these steps, the clinical trial landscape can evolve to meet the complexities of modern research, ultimately benefiting both sponsors and patients in the pursuit of groundbreaking medical advancements.
What is Risk-Based Monitoring (RBM) in clinical trials?
Risk-Based Monitoring (RBM) in clinical trials is an approach that focuses on detecting and mitigating threats to patient safety and data integrity by strategically directing monitoring efforts based on the specific risks associated with each study, rather than relying solely on extensive on-site inspections.
How does RBM differ from traditional monitoring methods?
Unlike traditional monitoring methods that often involve comprehensive source verification and frequent on-site inspections, RBM allocates resources more efficiently by concentrating on critical areas that require closer scrutiny and minimizing oversight in lower-risk sectors.
What is the current trend in the adoption of RBM in clinical trials?
As of 2025, approximately 88% of clinical trials are integrating at least one component of clinical trial risk-based monitoring, indicating a growing recognition of the need for adaptive monitoring strategies in modern clinical research.
What are the benefits of implementing RBM in clinical trials?
Implementing RBM improves the quality of clinical studies, accelerates study completion times, and facilitates quicker access to innovative treatments for patients. It can also reduce clinical study costs by as much as 30% while improving data quality and operational efficiency.
How do sponsors utilize Key Risk Indicators (KRIs) in RBM?
Sponsors use Key Risk Indicators (KRIs) and centralized monitoring tools to proactively identify potential issues in clinical trials, particularly in high-enrollment studies where conventional verification methods may be impractical due to the large volume of data generated.
What challenges are associated with the implementation of RBM?
Common challenges include insufficient training for staff on RBM principles and a lack of clear communication channels among stakeholders, which must be addressed to maximize the advantages of RBM.
Why is RBM becoming imperative in the clinical study landscape?
The adoption of RBM methodologies is becoming imperative to safeguard data integrity and ensure timely submissions in an evolving clinical study landscape, transforming operational constraints into opportunities for enhanced management.