


The article emphasizes the critical objectives, design, and best practices of Phase II clinical trials, which play a vital role in evaluating the effectiveness of new therapies following initial safety assessments. These trials typically involve 100 to 300 participants and employ randomized controlled designs to rigorously assess treatment efficacy.
Notably, nearly 33% of medications advance to the next phase, highlighting the necessity for a robust methodology and strict adherence to ethical standards throughout the research process. This focus not only underscores the importance of these trials but also sets the stage for future advancements in clinical research.
Clinical trials are the backbone of medical innovation, meticulously designed to assess the safety and efficacy of new treatments that can transform patient care. Among these, Phase II trials hold particular significance, bridging the gap between initial safety assessments and broader efficacy evaluations. These trials ultimately shape the future of healthcare. However, the journey to successful Phase II trials is fraught with challenges, ranging from recruitment hurdles to complex regulatory requirements.
How can researchers effectively navigate this intricate landscape to ensure that their trials not only meet their objectives but also contribute meaningfully to medical advancements?
Clinical studies represent meticulously structured research investigations designed to evaluate the safety and effectiveness of new medical interventions, including drugs, devices, and treatment protocols. They play a pivotal role in the medical research landscape by providing essential evidence that supports the approval and utilization of new therapies. Through systematic evaluation of hypotheses in regulated settings, research studies assess the effectiveness and safety of treatments for human application. The results of these tests can lead to groundbreaking improvements in healthcare, offering renewed hope for patients with previously untreatable conditions.
Furthermore, research studies are essential for regulatory authorities, such as the FDA, enabling informed decisions about the authorization of new treatments. This process ensures that only safe and effective therapies are introduced to the market, ultimately enhancing patient care and contributing to the ongoing evolution of medical science. However, it is crucial to acknowledge that approximately 80% of medical studies fail to meet initial enrollment objectives, highlighting the significant challenges faced in achieving successful outcomes. As noted by research specialist Matej Mikulic, the likelihood of success in phase two studies is below 50 percent across nearly every disease domain, underscoring the intrinsic challenges of the research process.
Moreover, the typical cost of developing a medication in the U.S. is around $2.6 billion, illustrating the financial risks associated with these studies. Generally, it takes 10 to 15 years to create a new medication, emphasizing the lengthy and intricate nature of the research process. Additionally, the increasing complexity of medical studies, as observed in recent years, adds another layer of difficulty to the research environment.
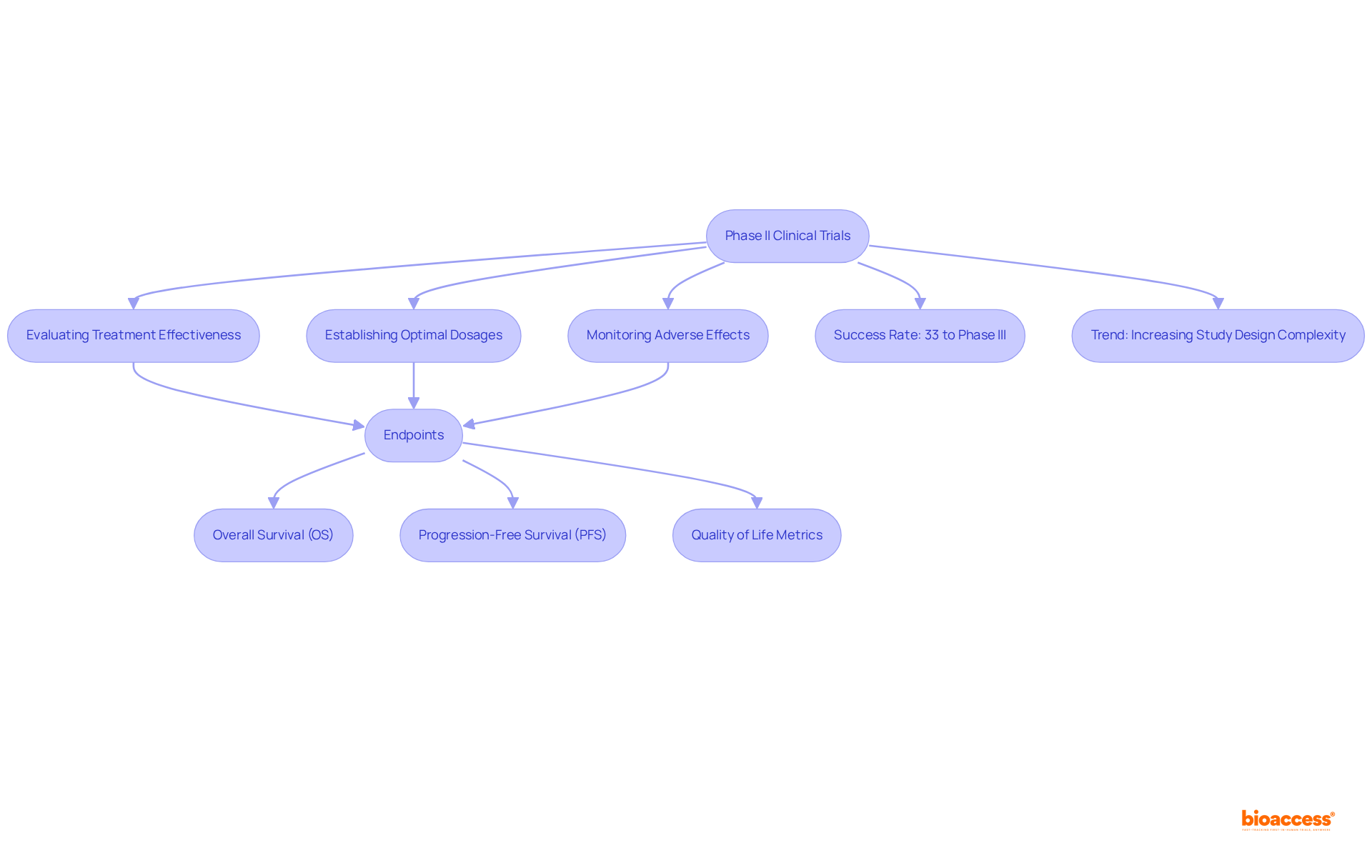
Clinical trials phase II play a pivotal role in evaluating the effectiveness of new therapies after the safety confirmations achieved in Stage I studies. Typically involving 100 to 300 participants, these studies are meticulously designed to ascertain the treatment's beneficial effects on specific diseases or conditions. The structure of Stage II studies frequently employs randomized controlled trials (RCTs), contrasting the new treatment with a placebo or standard care, thereby minimizing bias and enhancing the reliability of outcomes.
The primary objectives of clinical trials phase II encompass:
Additionally, these studies may explore various endpoints, such as overall survival (OS), progression-free survival (PFS), and quality of life metrics, to deliver a comprehensive understanding of the treatment's impact. Notably, nearly 33% of medications in clinical trials phase II progress to the subsequent stage, with approximately 50% of evaluations in clinical trials phase II proving successful, underscoring the significance of this phase in the medication development process.
Recent trends reveal a shift towards more intricate study designs, including adaptive formats that permit modifications based on interim data, thereby enhancing efficiency and flexibility. The average number of participants in clinical trials phase II has remained stable, typically ranging from several dozen to a few hundred, facilitating a thorough evaluation of treatment effectiveness and safety while remaining manageable for researchers. Furthermore, the average count of eligibility requirements per study has increased from 31 between 2001 and 2005 to 50 from 2011 to 2015, highlighting the growing complexity of study designs. As the landscape of medical studies evolves, understanding these design factors is essential for improving outcomes and advancing healthcare.
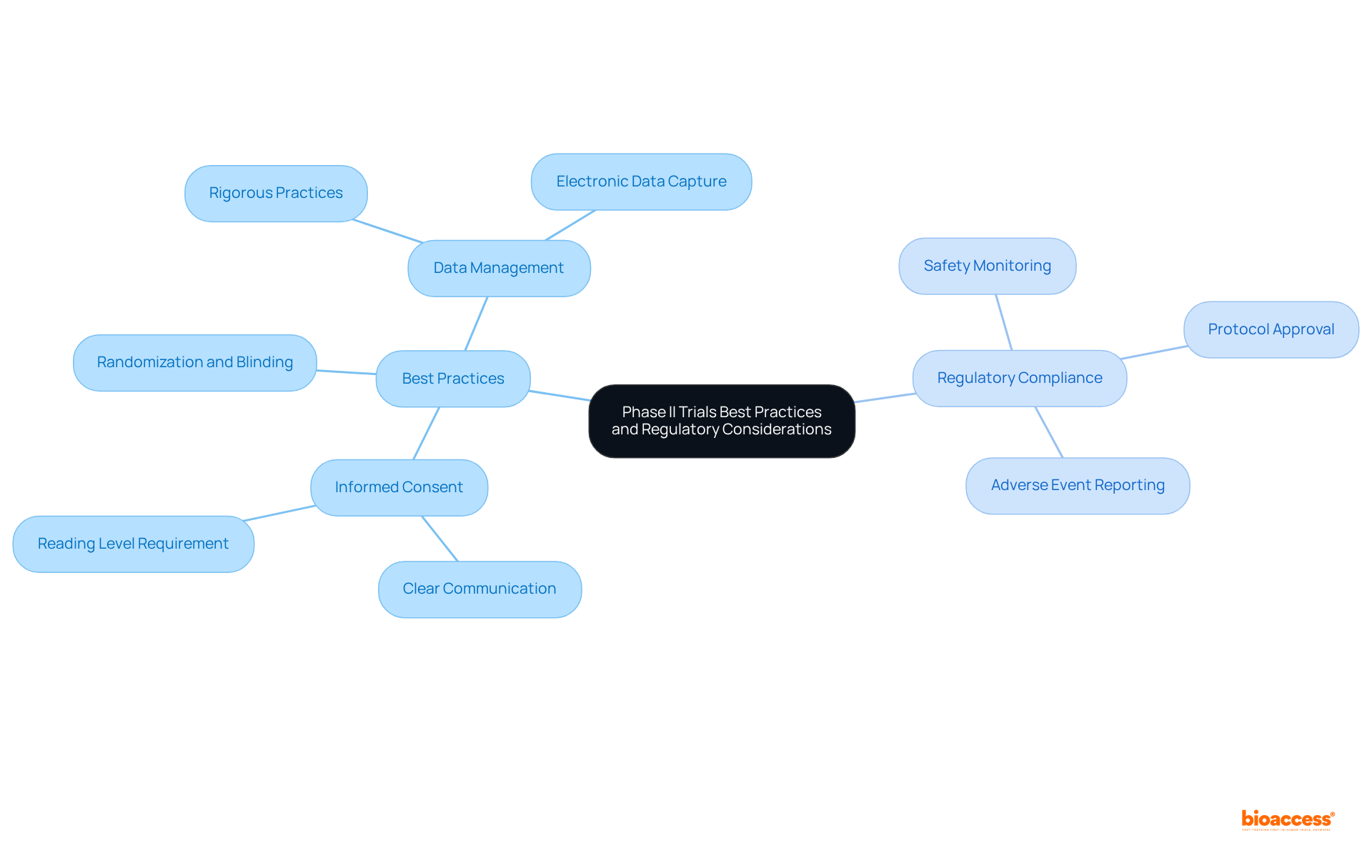
Applying best practices in clinical trials phase II is essential for guaranteeing ethical and scientific integrity. Adherence to Good Clinical Practice (GCP) guidelines is paramount, encompassing essential elements such as:
Notably, patients who do not read informed consent documents are nearly three times more likely to refuse participation in research, emphasizing the necessity of clear communication in improving participation rates.
Regulatory compliance is equally crucial; studies must conform to both local and international regulations, including those set by the FDA and EMA. This involves:
Statistics suggest that adherence to GCP guidelines greatly influences study outcomes, with compliance rates in clinical trials phase II demonstrating a dedication to ethical standards. For instance, a recent survey revealed that 85% of participants endorsed the creation of guidance on Statistical Analysis Plans (SAPs), underscoring the industry's emphasis on transparency and reproducibility in research.
Furthermore, promoting open dialogue with stakeholders—including regulatory bodies and ethics committees—is crucial for upholding trust and ensuring the study's integrity. Case studies, such as the rise in health research participation in England, demonstrate that organizations prioritizing stakeholder engagement often experience smoother regulatory processes and improved participant recruitment. By following these best practices, researchers can not only enhance the reliability of their findings but also contribute significantly to the advancement of medical knowledge and the development of new therapies. Additionally, bioaccess® enables enrollment 50% faster than traditional markets, showcasing the efficiency gained through proper compliance and practices.
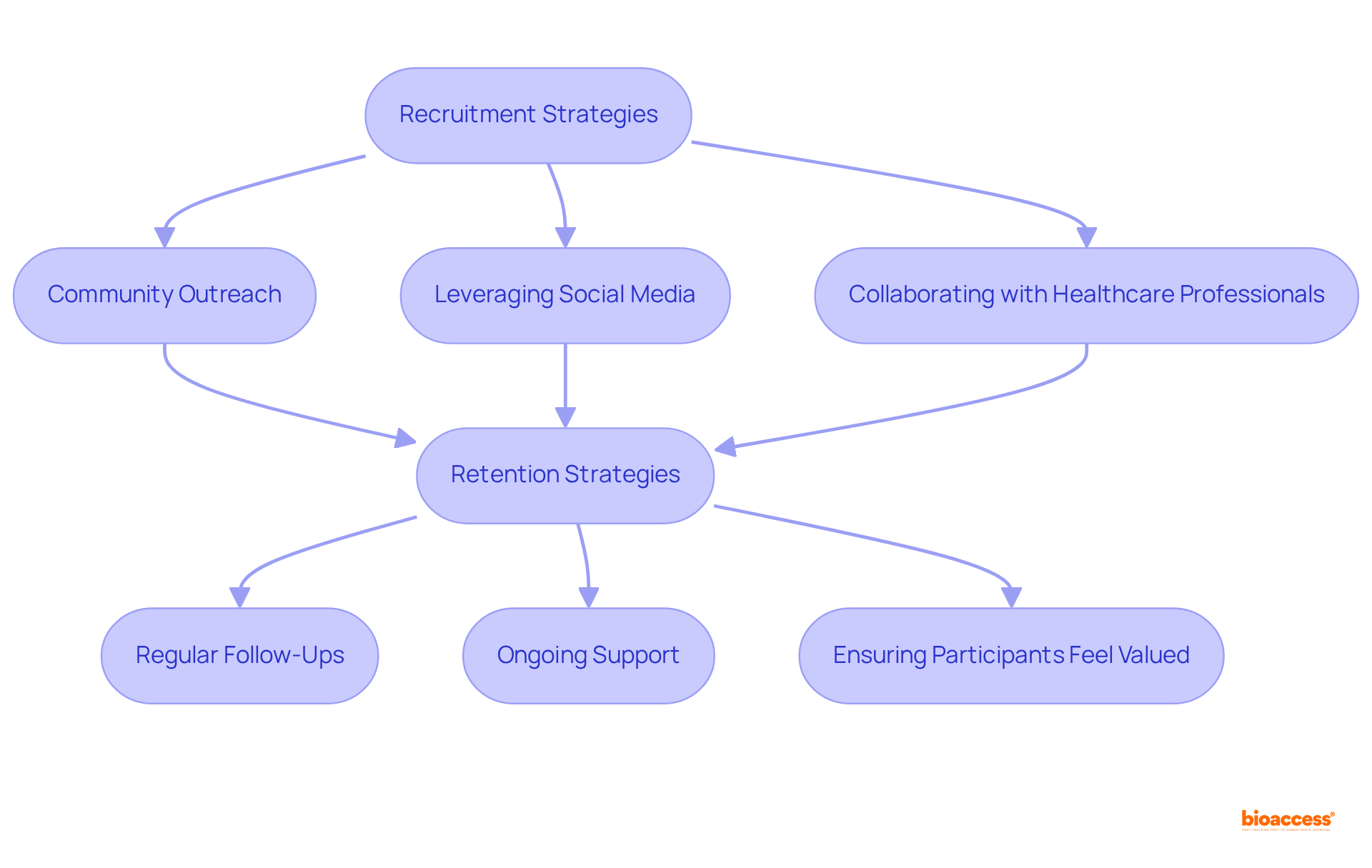
Enrolling and retaining patients are critical for the success of clinical trials phase II, as dropout rates can range from 15% to 40%. Effective hiring strategies involve:
Notably, 73% of patients prefer to learn about research opportunities through their doctor's office. Clear communication about the study's purpose, procedures, and potential benefits is essential to motivate individuals to participate.
Once enrolled, retaining participants becomes equally vital; this can be accomplished through:
Addressing barriers to participation, such as transportation or financial concerns, is crucial, especially considering that 70% of potential participants reside more than two hours away from a study center. Research indicates that 80% of clinical trials phase II face delays due to recruitment challenges, which underscores the need for proactive strategies.
By implementing these approaches, researchers can cultivate a robust participant pool, ultimately leading to more reliable and generalizable study outcomes. Furthermore, integrating patient-focused solutions, such as telemedicine and wearable devices that consistently track health metrics for instant feedback, can enhance engagement and retention, making the experience more accessible and supportive for participants.
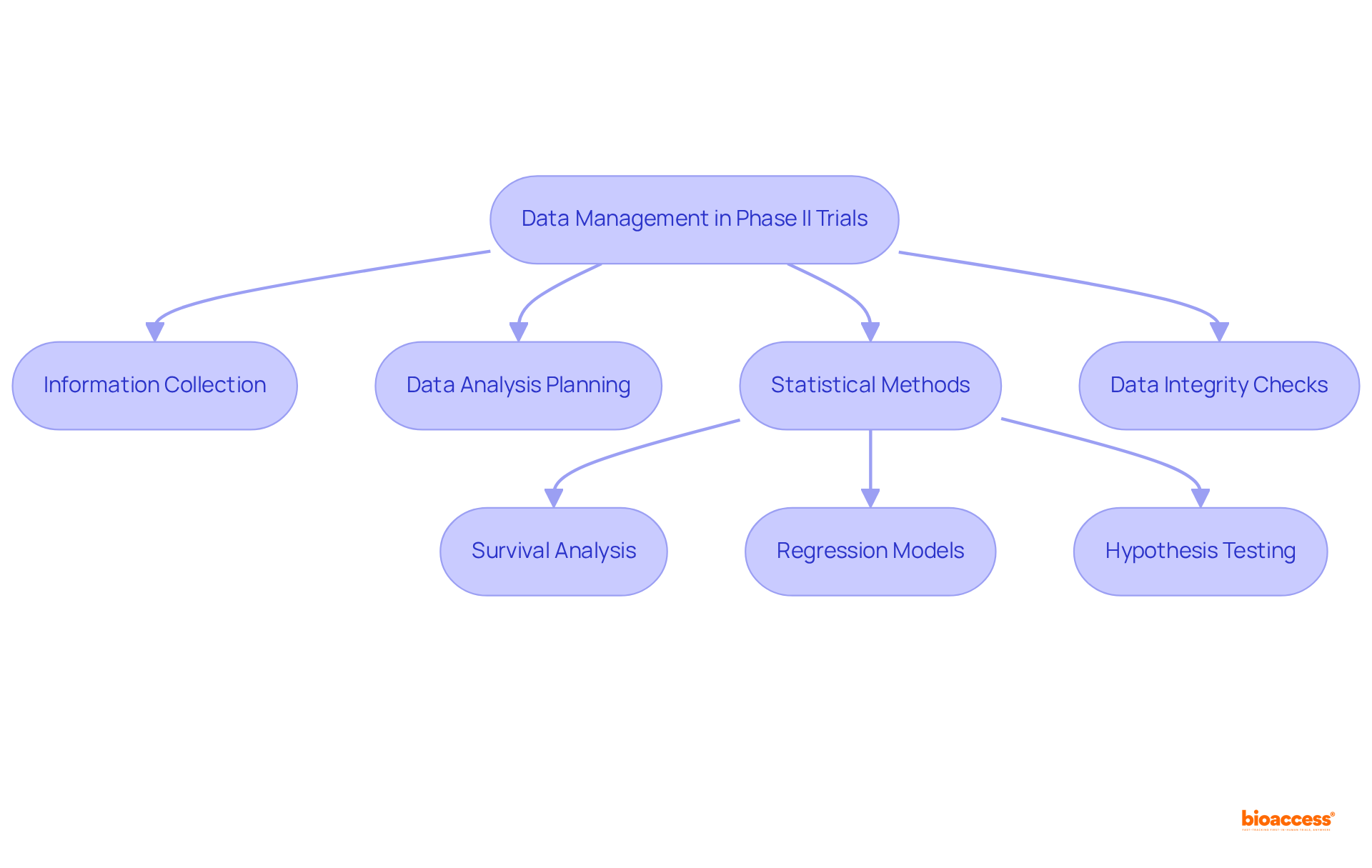
Efficient information management and analysis are crucial in clinical trials phase ii, as they ensure the integrity and reliability of results. Establishing robust information collection methods and utilizing electronic information capture systems are essential for maintaining accuracy, supported by regular audits. The Clinical Information Management (CIM) team plays a pivotal role in overseeing information gathering and ensuring adherence to protocols, which is vital for effective management.
Careful planning of statistical analysis is paramount, with methodologies such as:
commonly employed to evaluate treatment efficacy and safety. Pre-specifying endpoints and analysis plans is vital to mitigate bias and enhance transparency. Moreover, integrated, automated information management workflows can finalize patient enrollment 30% quicker and achieve database lock in 45% less time compared to fragmented systems.
By prioritizing these aspects—including preserving information completeness and securing sensitive patient details—researchers can significantly enhance the credibility of their findings, facilitating informed decision-making throughout the drug development process. Additionally, implementing end-to-end data management solutions can increase the likelihood of regulatory approval by 23%, underscoring the importance of robust data management practices.
Mastering Phase II clinical trials is essential for advancing medical research and enhancing patient care. This stage serves as a critical bridge between the initial safety assessments of Phase I and the more extensive efficacy evaluations of Phase III. By understanding the objectives, design, and best practices associated with Phase II trials, researchers can significantly contribute to the development of effective therapies that meet the needs of patients.
The article outlined the key objectives of Phase II trials, including:
It emphasized the importance of rigorous study design, including the use of randomized controlled trials, to ensure reliable outcomes. Furthermore, adherence to best practices and regulatory standards is vital for maintaining the integrity of the research process. Effective patient recruitment and retention strategies, alongside robust data management and analysis, were highlighted as crucial elements that influence the success of these trials.
Reflecting on the insights provided, it is clear that the success of Phase II clinical trials not only impacts the development of new therapies but also shapes the future of healthcare. Researchers and stakeholders must prioritize ethical practices, transparent communication, and innovative strategies to overcome challenges in recruitment and data management. By doing so, they will foster a research environment that not only advances medical knowledge but also ultimately improves patient outcomes and quality of life.
What are clinical trials and why are they important in medical research?
Clinical trials are structured research investigations designed to evaluate the safety and effectiveness of new medical interventions, including drugs and treatment protocols. They are crucial for providing evidence that supports the approval and use of new therapies, leading to improvements in healthcare and enabling regulatory authorities to make informed decisions about new treatments.
What challenges do clinical trials face?
Approximately 80% of medical studies fail to meet initial enrollment objectives, and the likelihood of success in phase two studies is below 50% across nearly every disease domain. Additionally, the average cost of developing a medication in the U.S. is around $2.6 billion, and it typically takes 10 to 15 years to create a new medication.
What is the role of phase II clinical trials?
Phase II clinical trials evaluate the effectiveness of new therapies after safety confirmations from Stage I studies. They typically involve 100 to 300 participants and are designed to assess treatment benefits, establish optimal dosages, and monitor adverse effects.
How are phase II clinical trials structured?
Phase II trials often employ randomized controlled trials (RCTs), comparing the new treatment with a placebo or standard care to minimize bias and enhance reliability. They also explore various endpoints such as overall survival (OS), progression-free survival (PFS), and quality of life metrics.
What is the success rate of medications in phase II clinical trials?
Nearly 33% of medications in clinical trials phase II progress to the next stage, with about 50% of evaluations proving successful. This phase is significant in the medication development process.
What recent trends are observed in clinical trial designs?
There is a trend towards more intricate study designs, including adaptive formats that allow modifications based on interim data, enhancing efficiency and flexibility. Additionally, the average number of eligibility requirements per study has increased, indicating growing complexity in study designs.
How many participants are typically involved in phase II clinical trials?
Phase II clinical trials typically involve between several dozen to a few hundred participants, allowing for a thorough evaluation of treatment effectiveness and safety.